Introduction
Within a generally high marine biodiversity (Jackson and Johnson, Reference Jackson and Johnson2001; Buzas et al., Reference Buzas, Collins and Culver2002), bryozoan diversity in Cenozoic tropical latitudes was also very high (Di Martino et al., Reference Di Martino and Taylor2017, Reference Di Martino, Taylor and Portell2019). In the Caribbean, a notable increase of bryozoan species richness has been recorded since the Burdigalian (ca. 18 Ma) (Cheetham and Jackson, Reference Cheetham, Jackson, Jackson, Budd and Coates1996; Cheetham et al., Reference Cheetham, Sanner and Jackson1999; O'Dea et al., Reference O'Dea, Herrera-Cubilla, Fortunato and Jackson2004; Di Martino et al., Reference Di Martino and Taylor2017, Reference Di Martino, Jackson, Taylor and Johnson2018). However, reports of fossil bryozoans in tropical regions, especially in continental South America, remain sparse (Taylor et al., Reference Taylor, James, Bone, Kuklinski and Kyser2009; Zágoršek et al., Reference Zágoršek, Ramalho, Berning and Távora2014; Taylor and Waeschenbach, Reference Taylor and Waeschenbach2015; Ramalho et al., Reference Ramalho, Serrano, Rueda, Távora, Zágoršek, Schmidt, Reid, Gordon, Walker-Smith and Percival2019). Diagenetic processes, which mainly affect bryozoans with aragonitic skeletons (Taylor et al., Reference Taylor, James, Bone, Kuklinski and Kyser2009), and several other factors, have undoubtedly biased against the fossil record of bryozoans in the Cenozoic tropics (Taylor and Di Martino, Reference Taylor, Di Martino, Rosso, Wyse-Jackson and Porter2014). In addition to the limited collection effort, small bryozoan colonies tended to be overlooked in the field and inventories are therefore largely incomplete. Consequently, taxonomic studies of bryozoan faunas from the early Miocene are generally limited not only for the Caribbean/Gulf of Mexico region (e.g., Sandberg, Reference Sandberg1962; Cheetham et al., Reference Cheetham, Sanner and Jackson1999, Reference Cheetham, Sanner and Jackson2007; Herrera-Cubilla and Jackson, Reference Herrera-Cubilla and Jackson2014; Di Martino et al., Reference Di Martino and Taylor2017), the western Atlantic (e.g., Zágoršek et al., Reference Zágoršek, Ramalho, Berning and Távora2014; Ramalho et al., Reference Ramalho, Távora, Tilbrook and Zágoršek2015, Reference Ramalho, Távora and Zágoršek2017, Reference Ramalho, Taylor, Moraes, Moura, Amado-Filho and Bastos2019), or the Indo-Pacific (e.g., Li, Reference Li1990; Guha and Gopikrishna, Reference Guha and Gopikrishna2005, Reference Guha and Gopikrishna2007; Di Martino and Taylor, Reference Di Martino and Taylor2014, Reference Di Martino and Taylor2015), but also for the more thoroughly investigated Paratethys/Mediterranean Sea (e.g., Duvergier, Reference Duvergier1920; Buge, Reference Buge1973; Nikulina and Taylor, Reference Nikulina and Taylor2010; Vávra, Reference Vávra, Ernst, Schäfer and Scholz2012; Di Martino and Taylor, Reference Di Martino and Taylor2017).
Despite their small size, bryozoans are a diverse group of invertebrates within marine benthic communities. Their distribution and species richness are related, in part, to the availability of hard substrata and habitat heterogeneity (Clarke and Lidgard, Reference Clarke and Lidgard2000). Coral reefs provide diverse surfaces for the settlement and growth of bryozoans, including cryptic habitats, such as caves, crevices, areas under coral colonies, rubble, and sand grains (Jackson and Winston, Reference Jackson and Winston1982; Choi, Reference Choi1984; Ramalho et al., Reference Ramalho, Taylor, Moraes, Moura, Amado-Filho and Bastos2018). In addition, a high diversity of macroinvertebrates (e.g., sponges, crabs, and mollusk shells) are susceptible to colonization by bryozoans (e.g., Almeida et al., Reference Almeida, Souza, Menegola and Vieira2017; Di Martino et al., Reference Di Martino, Taylor and Portell2019). Although bryozoans are not the most abundant organisms in these tropical ecosystems in terms of biomass, their species diversity is relatively high (e.g., Santodomingo et al., Reference Santodomingo, Novak, Pretković, Marshall, Di Martino, Giudice-Capelli, Rösler, Reich, Braga, Renema and Johnson2015).
Several studies have focused on the characterization of bryozoans associated with modern coral reefs in the western central Atlantic region, and have addressed the ecological roles they play in these ecosystems (Cuffey and Kissling, Reference Cuffey and Kissling1973; Schopf, Reference Schopf1974; Cuffey and Fonda, Reference Cuffey and Fonda1977; Jackson and Winston, Reference Jackson and Winston1982; Choi, Reference Choi1984; Winston, Reference Winston1984, Reference Winston1986; Winston and Jackson, Reference Winston and Jackson1984; Jackson et al., Reference Jackson, Winston and Coates1985). However, comprehensive taxonomic studies of bryozoan faunas in ancient coral reefs remain scarce.
The most representative taxonomic works on Caribbean Miocene bryozoans were carried out by Canu and Bassler (Reference Canu and Bassler1919, Reference Canu and Bassler1923) in the islands of Jamaica, Hispaniola, and Cuba, as well as in Costa Rica and the federal states of Maryland, Virginia, North Carolina, and Florida (USA); by McGuirt (Reference McGuirt1941) in Louisiana; by Scolaro (Reference Scolaro1968) and Di Martino et al. (Reference Di Martino and Taylor2017) in Florida; and by Cheetham et al. (Reference Cheetham, Sanner and Jackson1999) in the Dominican Republic and Panama. In Colombia, studies on fossil bryozoans are almost non-existent. Some work has been done on Devonian and Cretaceous samples (McNair, Reference McNair1940; Jerez-Jaimes et al., Reference Jerez-Jaimes, Cetina-Tarazona and Araque-Gomez2013), and there are isolated mentions of “Sertella sp.” and “Acanthodesia savartii form texturata” from the Miocene reefs in the oceanic island of Providencia (Buge in Geister, Reference Geister1992).
The purpose of this paper is to: (1) describe the bryozoan species associated with shallow coral reefs in the Siamaná Formation (Aquitanian, ca. 23–20 Ma), thereby contributing to knowledge of bryozoan assemblages in the Caribbean Basin; (2) estimate the role played by bryozoans in reefs from the Siamaná Formation; and (3) identify the biogeographical patterns of the bryozoan fauna during the early Miocene in the southern Caribbean.
Geologic setting
The Siamaná Formation crops out in the La Guajira Peninsula (northeast Colombia), in the northeastern foothills of Serranía de Cocinas, south of Serranía de Jarara and west of Serranía de Macuira (Fig. 1) (Rollins, Reference Rollins1965). The formation consists of conglomerates, sandstones, and fossiliferous limestones (Renz, Reference Renz1960). This formation is a diachronic sedimentary unit ranging from the late Oligocene to the early Miocene (Duque-Caro, Reference Duque-Caro1974; Silva-Tamayo et al., Reference Silva-Tamayo, Lara, Nana Yobo, Erdal, Sanchez and Zapata-Ramírez2017). Although the age of the upper limit of the Siamaná Formation continues to be a subject of study (Jaramillo et al., Reference Jaramillo, Sepulchre, Cardenas, Correa-Metrio, Moreno, Trejos, Vallejo, Hoyos, Martínez, Carvalho, Escobar, Oboh-Ikuenobe, Prámparo and Pinzón2020), the presence of corals, such as Siderastrea siderea (Ellis and Solander, Reference Ellis and Solander1786) (Flórez et al., Reference Flórez, Zapata-Ramírez and Klaus2019b, p. 427), bivalves, such as Mimachlamys canalis (Brown and Pilsbry, Reference Brown and Pilsbry1913) (Hendy et al., Reference Hendy, Jones, Moreno, Zapata and Jaramillo2015, p. 50), and principally the large benthic foraminifera assemblages found in the studied localities (W. Renema, personal communication, 2015) point to an early Miocene age. The early Miocene beds are mainly shallow-water marine carbonates deposited over the basement rocks of the Cocinas, Jarara, and Macuira paleoislands (Rollins, Reference Rollins1965, fig. 19.7; Bloch et al., Reference Bloch, Woodruff, Wood, Rincon, Harrington, Morgan, Foster, Montes, Jaramillo, Jud, Jones and MacFadden2016, fig. 4). Coral reefs grew fringing the paleoislands (Rollins, Reference Rollins1965; Flórez et al., Reference Flórez, Zapata-Ramírez and Klaus2019a, Reference Flórez, Zapata-Ramírez and Klausb). Deep marine siliciclastic sediments of the Uitpa Formation (Aquitanian–Burdigalian) unconformably overlie the Siamaná Formation (Renz, Reference Renz1960; Hendy et al., Reference Hendy, Jones, Moreno, Zapata and Jaramillo2015; Moreno et al., Reference Moreno, Hendy, Quiroz, Hoyos, Jones, Zapata, Zapata, Ballen, Cadena, Cárdenas, Carrillo-Briceño, Carrillo, Delgado-Sierra, Escobar, Martínez, Martínez, Montes, Moreno, Pérez, Sánchez, Suárez, Vallejo-Pareja and Jaramillo2015). Details about the stratigraphy of the collecting localities are provided in Flórez et al. (Reference Flórez, Zapata-Ramírez and Klaus2019a, fig. 2).

Figure 1. Locality maps. (1) Location of the La Guajira Peninsula in the Caribbean region with detail of the sampled zone showing Serranía de Cocinas (SC), Serranía de Jarara (SJ), and Serranía de Macuira (SM) surrounding the Cocinetas Basin (CB) (box), and including the locality La Flor de La Guajira (station 550002). (2) Close-up of localities Arroyo Ekieps (stations 550008, 550011, 550012, 550013) and Arroyo Uitpa (stations 550005, 550006) at the foothills of the of Serranía de Jarara in the Cocinetas Basin.
Materials and methods
The studied material was collected in the coral reef facies of the Siamaná Formation during two expeditions to the Cocinetas Basin carried out in 2011 and 2014. The bryozoan specimens were obtained from the surfaces of coral colonies and in the attached sediment. The coral samples were collected from seven stations along transects of 10 m (Fig. 1; Table 1). These were washed and scrubbed with a soft brush; the residual sediment was wet-sieved over mesh-sizes of 250 and 63 μm. In some cases, the sediment was gently removed from the corals with a needle and a paintbrush to preserve erect bryozoan fragments. To separate the encrusting colonies from the large coral substrates, when possible, small fragments of the corals were cut using a motor tool. Bryozoan specimens were cleaned using an ultrasonic bath for a few seconds to a few minutes, depending on the fragility of the colony. Coated and uncoated specimens were examined with scanning electron microscopy (SEM) at the “Centro de Instrumentación Científica, Universidad de Granada,” employing FEI Quanta 400 and FEI Qemscan 650F microscopes operating at low- and high-vacuum modes using backscattered and secondary electron detectors. Measurements of zooidal characters were made from SEM images using the image-processing program ImageJ (https://imagej.nih.gov/ij) and are given in mm, including mean (X), observed range (R), standard deviation (SD), and the number of measurements (N) (see tables for each species). The systematic paleontology section of Cheilostomatida follows the interim classification compiled by D.P. Gordon for the Treatise on Invertebrate Paleontology (Reference Gordon2011). A list of collected specimens included in this study with catalog numbers, locality names, station numbers, type of colony, substrate, and number of specimens available is provided in Appendix 1. Descriptions and illustrations of the ascophoran-grade cheilostomes mentioned in the “Remarks” will be part of a separate work currently in preparation.
Table 1. Siamaná Formation localities and stations studied in the present paper. Age is from Silva-Tamayo et al. (Reference Silva-Tamayo, Lara, Nana Yobo, Erdal, Sanchez and Zapata-Ramírez2017) and based on strontium isotopes in coralline algae.

Repositories and institutional abbreviations
The samples described and illustrated and the type specimens are stored in the reference collection of the Mapuka Museum of the Universidad del Norte, Barranquilla-Colombia (MUN-STRI). Type material used for comparative purposes is housed in the U.S. National Museum of Natural History, Washington, USA (USNM); Santa Barbara Museum of Natural History, Santa Barbara, USA (SBMNH); and Muséum national d'Histoire naturelle, Paris, France (MNHN).
Systematic paleontology
Order Cyclostomatida Busk, Reference Busk and MacGillivray1852a
Suborder Tubuliporina Milne-Edwards, Reference Milne-Edwards1838
Family Entalophoridae Reuss, Reference Reuss1869
Genus Mecynoecia Canu, Reference Canu1918
Type species
Entalophora proboscidea Milne-Edwards, Reference Milne-Edwards1838 from the Mediterranean Sea, Recent; by original designation.
Mecynoecia sp. indet.
Figure 2.1, 2.2; Table 2
Occurrence
Early Miocene, Siamaná Formation, Arroyo Ekieps, Colombia.
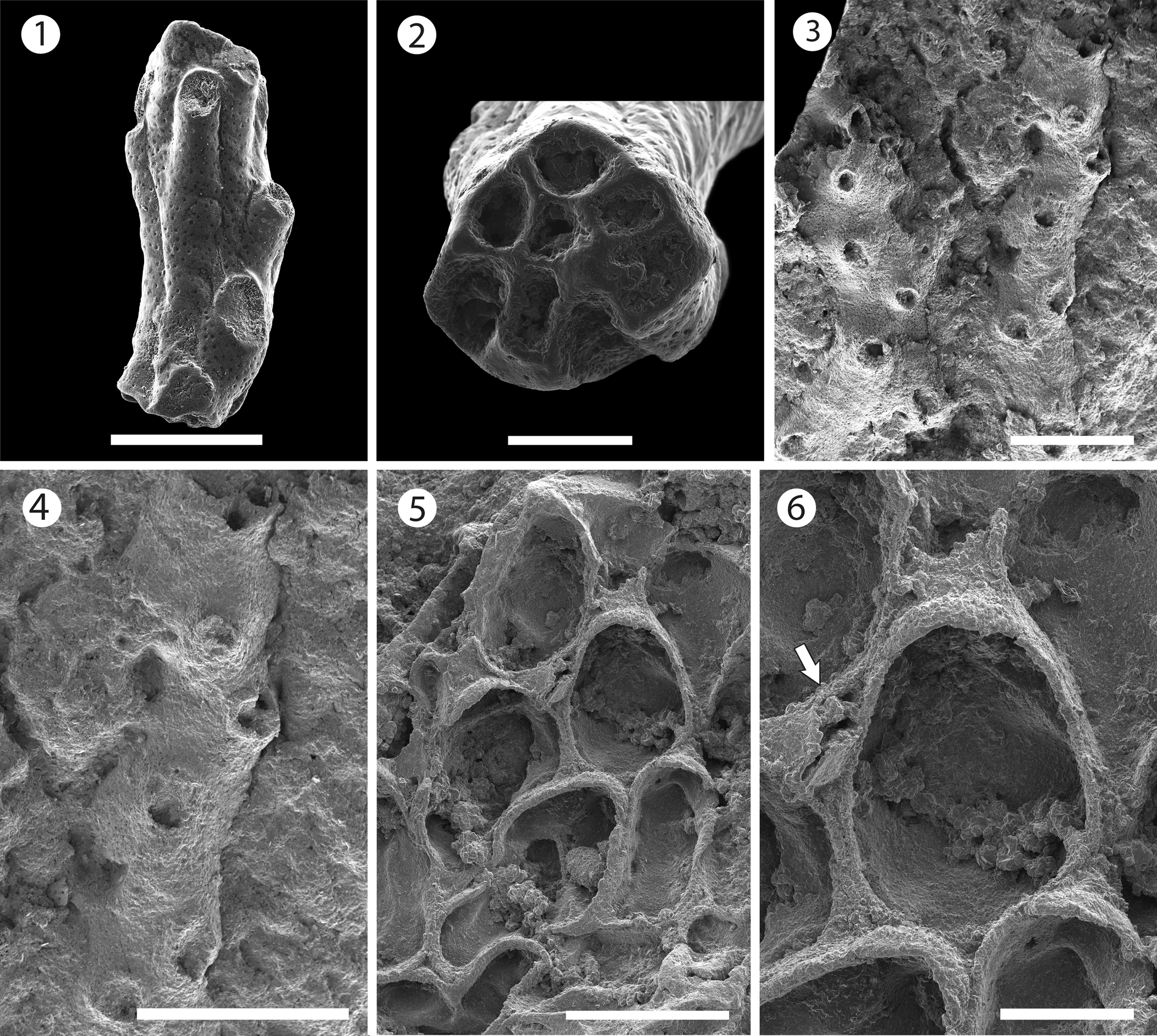
Figure 2. Mecynoecia sp. indet. (MUN-STRI-47623): (1) branch fragment; (2) view of the base of the branch fragment showing the absence of an axial lumen in cross-section. ?Oncousoecia sp. indet. (MUN-STRI-47625): (3) general view, (4) detail of the bifurcate branch. Copidozoum sp. indet. (MUN-STRI-47627): (5) general view of the colony, (6) detail of the zooid and avicularium (arrowed). All specimens are from the Siamaná Formation, Arroyo Ekieps locality. Scale bars are (1, 5) 0.5 mm; (2, 6) 0.2 mm; (3, 4) 1 mm.
Table 2. Measurements of Mecynoecia sp. indet. X = mean; R = observed range; SD = standard deviation; N = number of measurements.

Description
Colony erect with branches circular in cross section, ~0.46 mm in diameter. Zooids cylindrical with openings arranged in somewhat alternating whorls all around the branch, zooidal boundaries marked by narrow, thread-like, grooves. Peristomes not preserved. Frontal surface smooth, undulose, covered by moderately spaced, rounded pseudopores. Axial lumen absent. Gonozooids not observed.
Remarks
Canu and Bassler (Reference Canu and Bassler1920, p. 734) expanded the genus Entalophora Lamouroux, Reference Lamouroux1821 to include erect cyclostomes with cylindrical branches and zooids arranged in all directions and lacking gonozooids. However, Walter (Reference Walter1970) showed that species of the genus Entalophora were characterized by branches with a narrow axial lumen and subtriangular gonozooids. Based on the absence of the axial lumen, we place this poorly preserved, single branch fragment in Mecynoecia sp. indet. The lack of preserved gonozooids means that species-level identification is problematic. Mecynoecia sp. indet. was found in the sediment cemented to the scleractinian coral Goniopora hilli Vaughan, Reference Vaughan1919.
Family Oncousoeciidae Canu, Reference Canu1918
Genus Oncousoecia Canu, Reference Canu1918
Type species
Oncousoecia lobulata Canu, Reference Canu1918 from the British Isles, Recent; by subsequent designation (Taylor and Zatoń, Reference Taylor and Zatoń2008).
?Oncousoecia sp. indet.
Figure 2.3, 2.4; Table 3
Occurrence
Early Miocene, Siamaná Formation, Arroyo Ekieps, Colombia.
Table 3. Measurements of ?Oncousoecia sp. indet. X = mean; R = observed range; SD = standard deviation; N = number of measurements.

Description
Colony encrusting with large zooids, arranged in uni- to biserial alternating rows. Branches straight to slightly curved, apparently bifurcating. Zooids tubular (mean L/W = 1.66); zooidal boundaries not clearly demarcated. Zooidal surface densely perforated by rounded pseudopores, sometimes with transverse striations. Peristomes rising almost perpendicularly to the zooidal surface, with presumably circular to oval apertures. Gonozooids not observed.
Remarks
Based on the encrusting and apparently bifurcating colony form, as well as relatively widely spaced and oligoserially arranged autozooids with circular apertures (Taylor and Zatoń, Reference Taylor and Zatoń2008), we tentatively attribute this specimen to Oncousoecia. The specimen from the Siamaná Formation is an incomplete, poorly preserved colony. The broken peristomes and the absence of gonozooids prevent further identification. Oncousoecia has a long fossil record from the Early Jurassic to the Recent (Taylor and Zatoń, Reference Taylor and Zatoń2008). Five fossil species are known from North America: ?Oncousoecia nonomologabili Taylor and McKinney, Reference Taylor and McKinney2006 and O. khirar Martha, Taylor, and Rader, Reference Martha, Taylor and Rader2019 from the Cretaceous of New Jersey and Texas, respectively; O. contortilis (Lonsdale, Reference Lonsdale1845) and O. bifurcata (Ulrich and Bassler, Reference Ulrich, Bassler and Weller1907) from the Paleocene of New Jersey; and O. quinqueseriata Canu and Bassler, Reference Canu and Bassler1920 from the Oligocene of Alabama. In addition, three Recent species are also known: O. abrupta and O. ovoidea, both described by Osburn (Reference Osburn1953) from California, and O. arcuata Canu and Bassler, Reference Canu and Bassler1928 from the Gulf of Mexico. Among them, O. ovoidea, O. khirar, and ?O. nonomologabili differ from ?Oncousoecia sp. indet. in having fan-shaped colonies; O. bifurcata differs in having wider encrusting branches (1.2 mm vs. 0.66 mm), while O. quinqueseriata has narrower branches (0.5 mm), becomes erect, and has five longitudinal rows of autozooids. ?Oncousoecia sp. indet. resembles the early astogenetic stages of both O. contortilis and O. abrupta, but differs from the former species in having slightly curved instead of contorted branches, and from the latter Pliocene species in having wider branches (0.66 vs. 0.45 mm). Furthermore, it resembles O. arcuata in the shape of the branch and autozooidal arrangement, but differs in having a larger peristome diameter. ?Oncousoecia sp. indet. differs also from the European Miocene Oncousoecia biloba (Reuss, Reference Reuss1848) and Oncousoecia repens (Wood, Reference Wood1844). The former species has multiserial and unilamellar erect colonies (Zágoršek, Reference Zágoršek2003, Reference Zágoršek2010), while O. repens differs in having bi-, tri-, or multiserial branches (Busk, Reference Busk1859). ?Oncousoecia sp. indet. was found, along with the ascophoran-grade cheilostome bryozoan Poricella sp., encrusting a fragment of the scleractinian coral Alveopora tampae Weisbord, Reference Weisbord1973.
Order Cheilostomatida Busk, Reference Busk and MacGillivray1852a
Superfamily Calloporoidea Norman, Reference Norman1903
Family Calloporidae Norman, Reference Norman1903
Genus Copidozoum Harmer, Reference Harmer1926
Type species
Membranipora plana Hincks, Reference Hincks1880 from Australia, Recent; by original designation.
Copidozoum sp. indet.
Figure 2.5, 2.6; Table 4
Occurrence
Early Miocene, Siamaná Formation, Arroyo Ekieps, Colombia.
Table 4. Measurements of Copidozoum sp. indet. X = mean; R = observed range; SD = standard deviation; N = number of measurements.
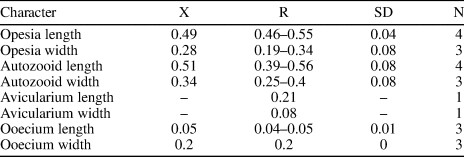
Description
Colony encrusting, unilaminar and multiserial. Zooids oval to irregular, distinctly separated by deep grooves (mean L/W = 1.49). Opesia occupying most of the frontal surface, same shape as the zooids. Gymnocyst reduced, visible only in some zooids proximally. Cryptocyst narrow, forming a thin and elevated mural rim. A single interzooidal avicularium observed, lozenge-shaped with narrow, rounded triangular rostrum, proximolaterally directed, seemingly with pivotal condyles. Spines absent. Ancestrula and ovicells not observed.
Remarks
Based on the characters of the interzooidal avicularium and autozooids, we place this specimen in Copidozoum. Other species of Copidozoum recorded in American Miocene deposits include C. parvirostris (Canu and Bassler, Reference Canu and Bassler1923) and C. tenuirostris (Hincks, Reference Hincks1880). Copidozoum sp. indet. differs from these species in having a thicker mural rim and in the orientation of the avicularia, which is distal both in C. parvirostris (Di Martino et al., Reference Di Martino, Taylor and Portell2019, p. 14, fig. 12) and C. tenuirostris (Osburn, Reference Osburn1950, pl. 7, fig. 4). Copidozoum sp. indet. also resembles Aplousina Canu and Bassler, Reference Canu and Bassler1927 in the general appearance of the autozooids; however, Aplousina lacks avicularia (Canu and Bassler, Reference Canu and Bassler1927, p. 3). Copidozoum sp. indet. was found growing on the basal surface of the scleractinian coral Colpophyllia willoughbiensis (Vaughan, Reference Vaughan1919), along with the microporid Calpensia caribensis n. sp. and the phidoloporid Rhynchozoon sp.
Family Antroporidae Vigneaux, Reference Vigneaux1949
Genus Antropora Norman, Reference Norman1903
Type species
Membranipora granulifera Hincks, Reference Hincks1880 from Madeira, Recent; by subsequent designation (Tilbrook, Reference Tilbrook1998).
Holotype
MUN-STRI-47628, from the early Miocene Siamaná Formation, Arroyo Uitpa, La Guajira, Colombia.

Figure 3. Antropora guajirensis n. sp. from the Siamaná Formation, Arroyo Uitpa (holotype MUN-STRI-47628): (1) general view; (2) detail of the autozooids with intramural reparative budding, drop shaped interzooidal avicularia, and cap-like ooecium (arrowed); (3) close-up of two autozooids and interzooidal avicularia. Scale bars are (1) 0.25 mm; (2) 0.2 mm; (3) 0.15 mm.
Table 5. Measurements of Antropora guajirensis n. sp. X = mean; R = observed range; SD = standard deviation; N = number of measurements.
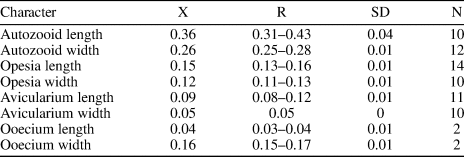
Diagnosis
Colony encrusting. Zooids oval to elliptical with oval to pear-shaped opesia. Gymnocyst smooth, developed proximally. Cryptocyst granular, surrounding the opesia, tapering distally. Interzooidal avicularia elliptical to drop-shaped, randomly placed, oriented distolaterally. Ooecium cap-like, imperforate.
Description
Colony encrusting, multiserial, and unilaminar. Zooids distinctly separated by deep grooves, oval to elliptical in shape (mean L/W = 1.37), sometimes polygonal where the proximal gymnocystal area is more extensive. Opesia oval to pear-shaped, occupying half to two-thirds of the frontal surface. Gymnocyst smooth, more developed proximally and surrounding the cryptocyst. Cryptocyst granular, extended proximally, narrowing progressively laterally, tapering distally. Avicularia interzooidal, elliptical to drop-shaped with rounded rostrum, oriented distolaterally, and arranged randomly within the colony. Ovicells endozooidal; ooecium small, cap-like, smooth, and imperforate. Intramural reparative budding common. Large vicarious avicularia not observed.
Etymology
Named after the La Guajira Peninsula, in reference to the region where it was collected for the first time, plus the Latin suffix -ensis, meaning originating in.
Remarks
Canu and Bassler (Reference Canu and Bassler1917, p. 17, 28) introduced the genera Membrendoecium and Dacryonella for some American fossil species; however, both genera have been regarded as junior synonyms of Antropora (Bassler, Reference Bassler and Moore1953; Tilbrook, Reference Tilbrook1998). Among the American fossil species of Antropora, the late Pliocene Antropora parvicapitata (Canu and Bassler, Reference Canu and Bassler1923) differs from A. guajirensis n. sp. in having small rounded kenozooids between the zooids and a single median tubercle in the proximal gymnocyst (Taylor and Foster, Reference Taylor and Foster1998, p. 66, figs. 5, 6; Di Martino et al., Reference Di Martino and Taylor2017, p. 108, figs. 8b, c). Antropora lowei (Canu and Bassler, Reference Canu and Bassler1920) from the Oligocene differs in having small, but frequent, indistinct “avicularia,” which in fact could be “interopesial cavities” between the zooids (Canu and Bassler, Reference Canu and Bassler1920, p. 121, pl. 81, fig. 1). The Recent A. typica Canu and Bassler (Reference Canu and Bassler1928) differs in having a pair of small interzooidal avicularia placed at the distal end of most autozooids and oriented distally (Tilbrook, Reference Tilbrook1998), while the new species has interzooidal avicularia oriented distolaterally, placed randomly. In the Siamaná Formation, it was found encrusting the rubble of Porites sp., and co-existing with the bryozoans Gymnophorella hadra n. gen. n. sp. and ?Hippopleurifera sp. 1.
Family Quadricellariidae Gordon, Reference Gordon1984
Genus Nellia Busk, Reference Busk and MacGillivray1852
Type species
Cellaria tenella (Lamarck, Reference Lamarck1816) from Austral seas (?), Recent; by subsequent designation (Busk, Reference Busk1852b).
Nellia cf. N. tenella (Lamarck, Reference Lamarck1816)
Figure 4.1–4.3; Table 6
- cf. 1816
Cellaria tenella Lamarck, p. 135.
- cf. 1920
Nellia oculata; Canu and Bassler, p. 196, pl. 82, figs. 6–10.
- cf. 1984
Nellia tenella; Winston and Cheetham, p. 257, figs. 1, 2.
- cf. 2017
Nellia tenella; Di Martino et al., p. 109, fig. 9.
- cf. 2019
Nellia tenella; Ramalho et al., p. 111, figs. 2a–c.
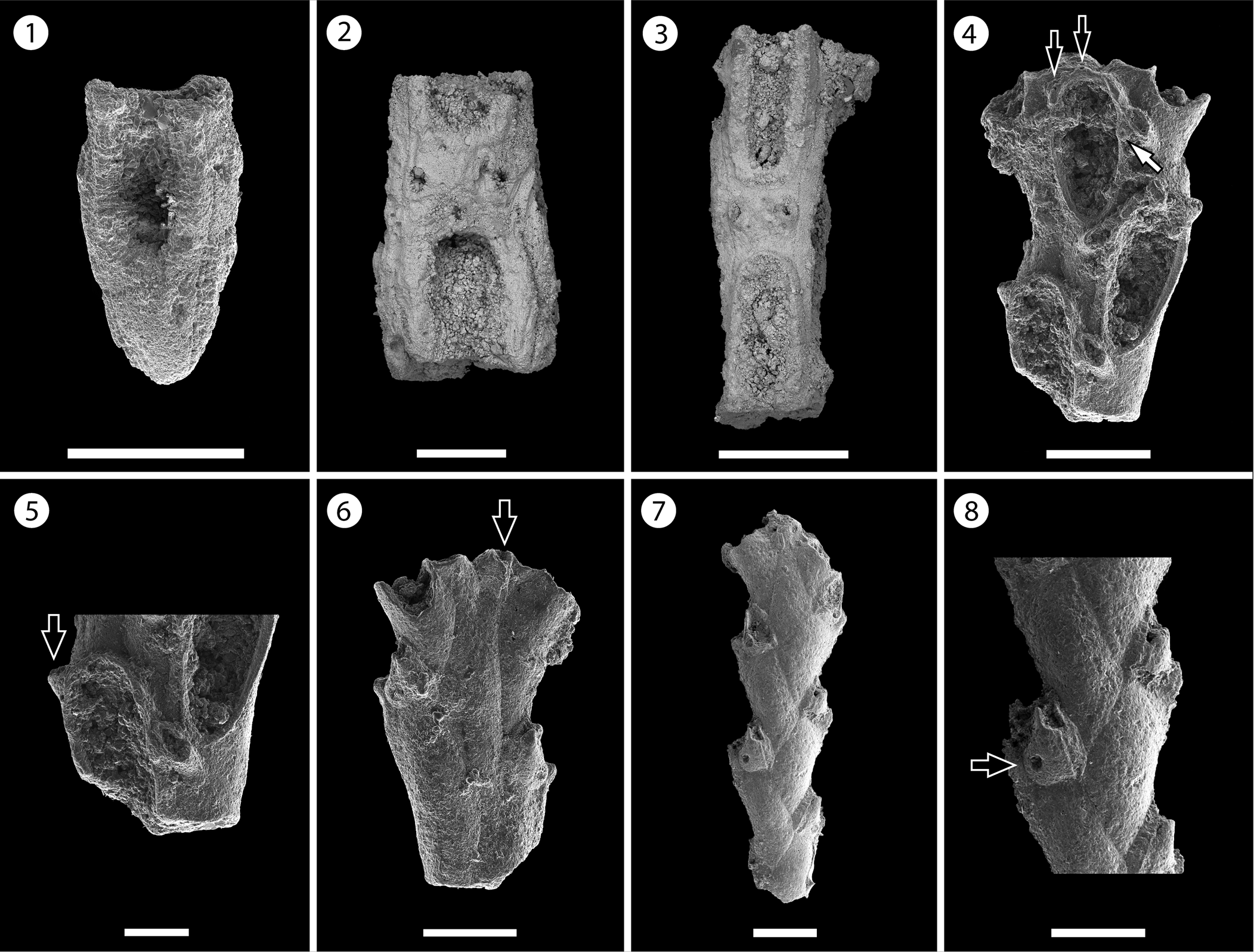
Figure 4. Nellia cf. N. tenella (Lamarck, Reference Lamarck1816) (MUN-STRI-47630): (1) part of a tapering zooid at the tip of the branch, (2) paired elliptical avicularia, (3) branch fragment with incomplete zooids. Licornia sp. indet. (MUN-STRI-47632): (4) frontal view of the branch at bifurcation showing the breaking point of the nodal joint crossing through the proximal part of the zooid, two distal spine bases (black arrows), and the insertion of the scutum (white arrow); (5) frontal view of the branch with the lateral (arrowed) and frontal avicularia; (6) abfrontal view of the branch at bifurcation showing the single axial vibraculum (arrowed); (MUN-STRI-47633): (7) abfrontal view of a branch fragment showing the vibracular chambers; (8) detail of the vibracular chamber showing the straight setal groove and the rhizoidal pore (black arrow). All specimens are from the Siamaná Formation, Arroyo Ekieps locality. Scale bars are (1, 3, 4, 6) 0.2 mm; (2, 8) 1 mm; (5) 0.1 mm; (7) 0.25 mm.
Table 6. Measurements of Nellia cf. N. tenella. X = mean; R = observed range; SD = standard deviation; N = number of measurements.

Syntypes
MNHN-IB-2008-4546, MNHN-IB-2008-5255, Southern seas (?), Recent.
Occurrence
?Late Cretaceous to Recent. The oldest record is from the Maastrichtian of Jamaica (Cheetham, Reference Cheetham1968). Eocene Crystal River Formation, Florida, USA (Winston and Cheetham, Reference Winston, Cheetham, Eldredge and Stanley1984). Eocene Upper Bracklesham Beds, England; France, Spain, and Senegal (Cheetham, Reference Cheetham1966). Oligocene Chickasawhay Formation, Alabama, USA (Winston and Cheetham, Reference Winston, Cheetham, Eldredge and Stanley1984). Early Miocene Pirabas Formation, Pará State, Brazil (Ramalho et al., Reference Ramalho, Serrano, Rueda, Távora, Zágoršek, Schmidt, Reid, Gordon, Walker-Smith and Percival2019) and Arroyo Ekieps, Siamaná Formation, Colombia. Late Miocene Cercado Formation, Dominican Republic (Winston and Cheetham, Reference Winston, Cheetham, Eldredge and Stanley1984). Miocene from Egypt and Australia (Cheetham, Reference Cheetham1966). Recent, widespread in tropical and warm-temperate waters (Cheetham, Reference Cheetham1966).
Description
Colony erect, articulated. Branches narrow, quadriserial, squared in cross-section. Zooids arranged in four longitudinal rows, in alternate positions with their lateral neighbors. Zooids distinct, delimited by a slender groove, all similar in size, elongate and sub-rectangular in shape. Opesia oval, occupying most of the frontal surface. Gymnocyst smooth, extended proximally and reduced laterally and distally. Cryptocyst smooth, forming a rim around the opesia. Mural rim slightly raised. Two small, elliptical avicularia placed in the proximal corners of the gymnocyst of each zooid, with a circular rootlet pore below the raised rostrum. Autozooids located at the tip of the internode tapering proximally. Ooecium not observed.
Remarks
The internode fragments available in our material are poorly preserved and lack ooecia, preventing species-level identification. However, they resemble Nellia tenella in the characteristic arrangement and shape of the autozooids and avicularia. The specimens from the Siamaná Formation are similar to those from the Miocene of the Dominican Republic figured by Winston and Cheetham (Reference Winston, Cheetham, Eldredge and Stanley1984, fig. 2D). However, the size of the opesia in these fragments is smaller than those of the Dominican specimens, as well as the syntype (MNHN-IB-2008-4546), and other specimens from different regions and ages (Cheetham, Reference Cheetham1963, Reference Cheetham1966; Winston et al., Reference Winston, Vieira and Woollacott2014; Di Martino et al., Reference Di Martino and Taylor2017). Although Winston and Cheetham (Reference Winston, Cheetham, Eldredge and Stanley1984) suggested that the variation in size is not significant, and proposed to consider N. tenella as a living fossil, based on the results of the statistical analyses performed on specimens ranging from the Late Cretaceous to the Recent, certain characters, which could reveal differences between localities and periods, such as ooecium morphology, have seldom been recorded. New fossil collections and phylogenetic studies using Recent material are needed to confirm its living-fossil status or to reveal that it is a species complex.
“Nellia tenella” was very common in the Miocene (Winston and Cheetham, Reference Winston, Cheetham, Eldredge and Stanley1984). Its Recent distribution is circumtropical to subtropical and it has been recorded on coral reefs (Winston, Reference Winston1986). In the Siamaná Formation, N. cf. N. tenella was found in the sediment cemented to the scleractinian coral Goniopora hilli, co-occurring with the bryozoan Poricellaria sp. (as also observed in other fossil and recent localities and environments, e.g., intertidal rocks; Winston and Cheetham, Reference Winston, Cheetham, Eldredge and Stanley1984), Margaretta sp., Ditaxiporina sp., and Mecynoecia sp.
Superfamily Buguloidea Gray, Reference Gray1848
Family Candidae d'Orbigny, Reference d'Orbigny1851
Genus Licornia van Beneden, Reference van Beneden1850
Type species
Acamarchis jolloisii Audouin, Reference Audouin and Audouin1826 from Red Sea, Recent; by original designation.
Licornia sp. indet.
Figure 4.4–4.8; Table 7
Occurrence
Early Miocene, Siamaná Formation, Arroyo Ekieps, Colombia.
Table 7. Measurements of Licornia sp. indet. X = mean; R = observed range; SD = standard deviation; N = number of measurements.

Description
Colony erect, articulated. Branches flat, elliptical in cross-section. Zooids distinct, biserial, alternately arranged, sub-rectangular in shape (mean L/W = 2.97). Opesia oval, with a raised mural rim, occupying two-thirds of the frontal surface. ?Two distal spine bases. Base of the scutum arising from the distal third of the inner side of the opesia, next to the frontal avicularium of the adjacent zooid. Gymnocyst smooth, well developed proximally, tapering laterally and distally around the opesia. Avicularia adventitious, frontal, and lateral; frontal avicularia placed proximally immediately below the rim of the opesia, monomorphic, frequent, with a proximally raised, rounded rostrum, proximolaterally directed towards the exterior; lateral avicularia smaller than frontal avicularia, located on the outer distal corner of each autozooid, triangular. Abfrontal surface smooth. Vibracular chamber trapezoidal with rhizoidal pore and straight setal groove, directed obliquely to the internodal axis, occupying two-thirds of the length of the vibracular chamber. Nodal joints crossing the proximal opesia of the outer zooids and below the opesia of the inner zooids at the bifurcation. Single axial vibraculum in the bifurcation, with straight setal groove and lacking a rhizoidal pore. Ooecium not observed. Scutum and spines not preserved.
Remarks
We place these specimens in the genus Licornia based on the position of the joint crossings, the straight rostrum of the lateral avicularia, the oblique setal groove of the vibracular chambers, and the single axial vibraculum. The closest allied genus, Paralicornia Vieira et al., Reference Vieira, Spencer-Jones, Winston, Migotto and Marques2014, differs in having an opesia occupying only half of the total length of the zooids and a shorter setal groove.
North American Cenozoic species of Licornia include Canu and Bassler's (Reference Canu and Bassler1920) L. cookei, L. milneri, and L. resseri, all known from the Oligocene. Licornia sp. indet. differs from these species in the morphology and size of the frontal and lateral avicularia: L. cookei has rounded lateral avicularia, L. milneri has very large and acuminate frontal avicularia, and L. resseri has very large lateral avicularia. Licornia regularis (Osburn, Reference Osburn1940), known in the Caribbean region from the Pleistocene to Recent, differs in having larger vibracular chambers reaching half of the total length of the zooids, frontal avicularia with triangular rostrum, lateral avicularia with serrated rostrum, and squatter zooids (Winston, Reference Winston2005, p. 27, figs. 63–68). Other Recent tropical Western Atlantic species, such as L. drachi (Marcus, Reference Marcus1955) and L. micheli (Marcus, Reference Marcus1955), differ from Licornia sp. indet. in having a larger opesia that covers almost the whole frontal surface of the zooids, and lateral avicularia of variable size, sometimes as long as the opesia.
The scarcity of material, as well as the absence of diagnostic features, such as ooecia, spines, and scuta, prevent species-level identification. In the Siamaná Formation, the fragments of Licornia sp. indet. were found in the sediment cemented to the coral Acropora panamensis Vaughan, Reference Vaughan1919, co-occurring with other erect bryozoans including Ditaxiporina sp., Catenicella sp., and Margaretta sp.
Superfamily Microporoidea Gray, Reference Gray1848
Family Microporidae Gray, Reference Gray1848
Genus Calpensia Jullien, Reference Jullien1888
Type species
Membranipora calpensis Busk, Reference Busk1854 from the Mediterranean Sea, Recent; by original designation.
Calpensia caribensis new species
Figure 5.1–5.3; Table 8
Holotype
MUN-STRI-47635. Paratypes: MUN-STRI-47634, MUN-STRI-47636. From the early Miocene Siamaná Formation, Arroyo Ekieps, La Guajira, Colombia.

Figure 5. Calpensia caribensis n. sp. from the Siamaná Formation, Arroyo Ekieps (holotype MUN-STRI-47635): (1) autozooids showing the semicircular opesia and the proximally raised opercular region, (2) close-up of autozooids showing paired opesiules, (3) general view of the colony. Atoichos magnus n. gen. n. sp. from the Siamaná Formation, Arroyo Ekieps (holotype MUN-STRI-47637); asterisks indicate putative avicularia: (4) group of autozooids, (5) oblique view showing the development and shape of the cryptocyst and the distal raised opesial margin (arrowed), (6) zooids showing well-preserved opesia with opesiular indentations and the distal opesial margin (arrowed). Scale bars are (1, 2, 4–6) 0.5 mm; (3) 1 mm.
Table 8. Measurements of Calpensia caribensis n. sp. X = mean; R = observed range; SD = standard deviation; N = number of measurements.

Diagnosis
Colony encrusting, multiserial. Zooids sub-rectangular with semicircular orifice, placed over the raised opercular region. Cryptocyst granular, densely perforated (>100 pseudopores). Two symmetrical, elliptical opesiules, placed proximolaterally to the orifice.
Description
Colony encrusting, multiserial, unilaminar. Zooids separated by a suture on a raised mural rim, elongate and sub-rectangular in shape (mean L/W = 1.58), widening at about mid-length and arranged in alternating, longitudinal rows. Orifice terminal, semicircular with a straight proximal margin; opercular region slightly developed. Cryptocyst flat proximally, depressed towards the opesiular area at about two-thirds of zooidal length, and raised distally; granular surface, densely and evenly perforated with up to ~110–120 circular pseudopores, absent in the area surrounding the opesia and between the opesia and the opesiules; pseudopores ~5 μm in diameter. Mural rim raised and crenulated. Two elliptical opesiules, longer than wide, equal in size, located proximolaterally at about two-thirds of zooidal length, ~0.14 mm below the opesia.
Etymology
From the Caribbean Sea, in reference to the biogeographic region that the new species inhabited, plus the Latin suffix -ensis meaning originating in.
Remarks
The specimens of Calpensia caribensis n. sp. found in the Siamaná Formation resemble specimens of the Recent Mediterranean Calpensia nobilis (Esper, Reference Esper1797). Calpensia nobilis is commonly found in the late Miocene to the Recent of the Mediterranean (Hayward and McKinney, Reference Hayward and McKinney2002; Moissette et al., Reference Moissette, Dulai, Escarguel, Kazmer, Mueller and Saint-Martin2007). Canu and Bassler (Reference Canu and Bassler1923) identified specimens of Calpensia as C. impressa (Moll, Reference Moll1803) in the Oligocene of the southern Caribbean, which were subsequently attributed to C. nobilis by Buge (Reference Buge1957). The new species is similar to Mediterranean specimens of C. nobilis in the size of the opesia and opesiules (Hayward and McKinney, Reference Hayward and McKinney2002, p. 31, fig. 13 a–c), as well as in the mean ratios of opesia length/zooid length (0.13 vs. 0.12), and opesia width/zooid width (0.36 vs. 0.39). However, C. caribensis n. sp. has squatter autozooids (zooid length 0.67 mm vs. 0.82 mm; zooid width 0.43 mm vs. 0.38 mm). Although Canu and Bassler (Reference Canu and Bassler1930) and Buge (Reference Buge1957) reported wide variations of the size of the zooids in this species, the mean ratio of zooid length/zooid width between our specimens and Recent material differs greatly (1.58 vs. 2.15), indicating a different zooidal shape. In addition, the Siamaná Formation specimens have a greater number of pseudopores in the cryptocyst (almost twice as many; mean of pores number 116 vs. 61). Based on these differences, as well as the spatial and temporal distance between the two populations, we introduce this new species. In the Siamaná Formation, Calpensia caribensis n. sp. was found encrusting coralline algae covering the coral Alveopora tampae and on the basal surface of the coral Colpophyllia willoughbiensis, while small detached fragments were scattered in the sediment associated with Goniopora hilli. In our material, Calpensia caribensis n. sp. co-occurs with Copidozoum sp., Gemelliporidra sp., and Rhynchozoon sp.
Family Onychocellidae Jullien, Reference Jullien1882
Genus Atoichos new genus
Type species
Atoichos magnus n. gen. n. sp., from Arroyo Ekieps, Colombia, early Miocene, Siamaná Formation; by monotypy.
Diagnosis
As the type species by monotypy.
Etymology
From the Greek privative prefix a- and toichos (masculine), meaning wall, alluding to the absence of a raised mural rim surrounding the autozooids.
Remarks
We place Atoichos n. gen. in the family Onychocellidae because of its extensive cryptocyst, the bell-shaped opesia with opesiular identations, the probable presence of vicarious avicularia, and the absence of a gymnocyst and spines (Taylor et al., Reference Taylor, Martha and Gordon2018). Atoichos n. gen. resembles in general appearance the genus Steraechmella Lagaaij, Reference Lagaaij1952, belonging to the family Microporidae Gray, Reference Gray1848, which is commonly allied with Onychocellidae (Taylor et al., Reference Taylor, Martha and Gordon2018). It differs in having wider opesia and, as in remaining genera of this family, in lacking a raised margin around the proximal two-thirds of the zooid. The poor preservation of the single specimen available prevents a detailed description of the morphology of the putative vicarious avicularia (i.e., opesia and rostrum), which could alternatively be narrower autozooids placed at row bifurcations where two regular zooids sometimes occupy the same width of the one preceding them in the row. However, as observed in some calloporids in the studies carried out by Cook (Reference Cook1968) on the Recent genus Crassimarginatella Canu, Reference Canu1900 and by Cheetham et al. (Reference Cheetham, Sanner, Taylor and Ostrovsky2006) on the fossil genus Wilbertopora Cheetham, Reference Cheetham1954, the morphology of avicularia is variable, some involving only slight modifications of the autozooids. In some onychocellids (e.g., Euritina Canu, Reference Canu1900) interzooidal avicularia are similar to autozooids, although smaller (Taylor et al., Reference Taylor, Martha and Gordon2018, p. 1676, fig. 10).
Atoichos magnus new species
Figure 5.4–5.6; Table 9
Holotype
MUN-STRI-47637, from the early Miocene Siamaná Formation, Arroyo Ekieps, La Guajira, Colombia.
Table 9. Measurements of Atoichos magnus n. gen. n. sp. X = mean; R = observed range; SD = standard deviation; N = number of measurements.

Diagnosis
Colony encrusting, multiserial, uni- to multilaminar. Zooids rhomboidal to elliptical, defined by a raised opesial margin distally and a deep groove proximolaterally. Opesia terminal, triangular to bell-shaped, as long as wide, occupying the distal third of the frontal surface; opesiular indentations shallow; proximal edge projected, straight to slightly curved. Cryptocyst extensive, granular, flat to slightly convex. Gymnocyst absent. Putative vicarious avicularia narrower than regular zooids; rounded triangular, commonly located at row bifurcations; opesia poorly preserved. Ovicells unknown.
Description
Colony encrusting, multiserial, uni- to multilaminar. Early astogenetic stages apparently fan-shaped. Zooids distinct, elongate, rhomboidal, to elliptical (mean L/W = 1.74), separated distally by a raised opesial margin, and a fine and deep groove proximo-laterally. Opesia terminal, wide, triangular to bell-shaped, as long as wide; borders raised forming a lateral furrow between the opesia margin and the adjacent zooids; opesiular indentations located at the proximolateral corners, slightly constricted laterally, proximal edge projecting distally with straight to slightly concave margin. Cryptocyst granular, occupying two-thirds of the frontal surface, flat to slightly convex, narrow laterally to the opesia. Putative vicarious avicularia narrower and slightly shorter than ordinary feeding zooids, rounded triangular, commonly located at longitudinal row bifurcations; opesia poorly preserved, ?oval, occupying more than half of the frontal surface, rostrum extended but broken, observed only twice, probably rounded (Fig. 5.4). Ovicells not observed.
Etymology
From the Latin magnus, large, in reference to the large size of the autozooids.
Remarks
The single colony found in the Siamaná Formation exhibits signs of dissolution. However, its morphological features are well preserved enough to allow comparisons with other genera and species in the family Onychocellidae. Atoichos magnus n. gen. n. sp. resembles species of Aechmella Canu and Bassler, Reference Canu and Bassler1917 and Aechmellina Taylor, Marta, and Gordon, Reference Taylor, Martha and Gordon2018. Aechmella differs in having smaller interzooidal avicularia, about half the width of the zooids with an acutely triangular rostrum, while Aechmellina differs in having frequent, small interzooidal avicularia with a pointed rostrum. Atoichos magnus n. gen. n. sp. also resembles Floridinella vicksburgica Canu and Bassler, Reference Canu and Bassler1917 in having a wide opesia, but differs in having kenozooids and avicularia with a triangular rostrum oriented laterally (Cook and Bock, Reference Cook and Bock2001, p. 547). In the Siamaná Formation, Atoichos magnus n. gen. n. sp. was found encrusting a mollusk shell, sharing the substrate with Glabrilaria sp. indet. and a poorly preserved cyclostome.
Genus Smittipora Jullien, Reference Jullien1882
Type species
Vincularia abyssicola Smitt, Reference Smitt1873 from Cuba and Florida, USA, Recent; by original designation.
Occurrence
Early Miocene, Siamaná Formation, Arroyo Uitpa, Colombia.

Figure 6. Smittipora sp. indet. from the Siamaná Formation, Arroyo Uitpa (MUN-STRI-47638): (1) general view of the colony, (2) two zooids and a vicarious avicularium with round rostrum, (3) close-up of autozooids and a fertile zooid (asterisk). Scale bars are (1) 1 mm; (2) 0.25 mm; (3) 0.5 mm.
Table 10. Measurements of Smittipora sp. indet. X = mean; R = observed range; SD = standard deviation; N = number of measurements.

Description
Colony encrusting, multiserial, unilaminar. Zooids defined by a raised rim, sub-hexagonal, rounded distally (mean L/W = 1.32). Opesia subterminal, bell-shaped, with proximolateral indentations outlining a tongue-like process medially, occupying half to one-third of the total length of the zooid. Cryptocyst extensive, depressed, flat, and coarsely granular. Vicarious avicularia symmetrical, similar in length to autozooids, but about half of their width, rounded rhomboidal; opesia reversed pear-shaped; cryptocyst granular. Ovicellate zooids with the distal edge formed by the ‘cryptocyst’ of the distal zooid; fertile zooids slightly longer than non-fertile autozooids.
Remarks
Among genera of the family Onychocellidae, our specimen fits better in the genus Smittipora because of the symmetrical and rounded rostrum of its avicularia (Taylor et al., Reference Taylor, Martha and Gordon2018, p. 1702). Although some similarities in the general morphology of the zooid can be found also with species of the genus Floridina Jullien, Reference Jullien1882, this latter genus differs in having acutely triangular rostra, as well as deep opesiular indentations, resulting in a more trifoliate opesia with lateral constrictions (Taylor et al., Reference Taylor, Martha and Gordon2018, p. 1678). Three species of Smittipora have been described from the North American Oligocene and two from the Miocene: S. fusiformis (Canu and Bassler, Reference Canu and Bassler1917), S. lineata (Canu and Bassler, Reference Canu and Bassler1920), S. tenuis (Canu and Bassler, Reference Canu and Bassler1920), S. levinseni (Canu and Bassler, Reference Canu and Bassler1917), and S. elongata (Canu and Bassler, Reference Canu and Bassler1923), respectively. The Oligocene species differ from Smittipora sp. indet. in having exclusively fusiform avicularia; S. levinseni, from the early Miocene to Recent, has smaller zooids and avicularia (Di Martino et al., Reference Di Martino, Taylor and Portell2017). Smittipora elongata from the late Miocene lacks proximolateral indentations and a tongue-like medial process on the opesia proximal border. Our specimen also resembles the Cretaceous species Reptolunulites zipfi Taylor and McKinney, Reference Taylor and McKinney2006 in having a bell-shaped opesia and symmetrical vicarious avicularia, but it differs in having a longer rostrum and a higher ratio between the length of the opesia and the length of the zooid. In the Siamaná Formation, Smittipora sp. indet. was found encrusting coralline algae covering the coral Porites sp., co-occurring with the ascophoran-grade cheilostome Hippopleurifera sp. and an undetermined cheilostome.
Family Steginoporellidae Hincks, Reference Hincks1884
Genus Gymnophorella new genus
Type species
Gymnophorella hadra n. gen. n. sp., from Arroyo Uitpa, Siamaná Formation, Colombia, early Miocene; by monotypy.
Diagnosis
As the type species by monotypy.
Etymology
From the Greek gymno-, referring to the gymnocystal calcification that can form in anascan-grade bryozoans in the frontal wall between the frontal membrane and the free edges of the vertical walls, and -phor meaning carrier, alluding to the presence of a gymnocyst in this Steginoporella-like genus, plus the suffix -ella commonly used in bryozoan names.
Remarks
Specimens of Gymnophorella n. gen. from the Siamaná Formation are similar to the fossil morphospecies “Steginoporella” cornuta (Osburn, Reference Osburn1950) recorded by Cheetham et al. (Reference Cheetham, Sanner and Jackson1999) from the Dominican Republic (ca. 7.1 Ma) and Panamanian Caribbean (ca. 3.5 and 1.8 Ma), as well as from the Recent Panamanian Pacific (figured in Cheetham and Jackson, Reference Cheetham, Jackson, Herrera-Cubilla and Jackson2000, fig. 2; NMiTA Database, 1996–2016 as aff. “Steginoporella” cornuta and “S.” cornuta; STRI Database, Reference Database2017 as S. cornuta), and to the Brazilian species Steginoporella evelinae Marcus, Reference Marcus1949 (see Winston et al., Reference Winston, Vieira and Woollacott2014, p. 147, fig. 5). They all have a narrow band of gymnocyst, more developed proximally, surrounding the cryptocyst and the opesia, a pair of conspicuous tubercles placed at the distal corners of the opesia, and similar shape of the opesia and opesiular indentations. However, in the original description of S. cornuta, Osburn (Reference Osburn1950, p. 108, pl. 12, figs. 3–6) made no mention of the presence of a gymnocyst, which is effectively absent in the holotype (SBMNH 635758, Fig. 7.1, 7.2), but present in a paratype (SBMNH 636427, Fig. 7.3, 7.4). In addition, Osburn (Reference Osburn1950) described B-zooids, also present in “Steginoporella” cornuta from the Pliocene of Panama (Cheetham and Jackson, Reference Cheetham, Jackson, Herrera-Cubilla and Jackson2000), but these were not observed or preserved in the material from the Siamaná Formation. On the other hand, the Siamaná specimens differ from S. evelinae in having an extended gymnocyst and an evenly granular cryptocyst. The family Steginoporellidae includes to date six genera, of which only Siphonoporella Hincks, Reference Hincks1880 develops a narrow gymnocyst, and has no avicularia or B-zooids (e.g., S. nodosa Hincks, Reference Hincks1880 and S. delicatissima [Busk, Reference Busk1861]). However, this genus differs from Gymnophorella n. gen. in having a lightly calcified skeleton and a very obvious polypide tube (Cook et al., Reference Cook, Bock, Gordon and Weaver2018, p. 96). Because Steginoporella sensu stricto lacks a gymnocyst (Harmer, Reference Harmer1926, p. 268; Gordon, Reference Gordon1984, p. 56), we introduce this new genus, and we place it in the family Steginoporellidae owing to its close resemblance with those (morpho-) species of Steginoporella and Siphonoporella discussed above. However, Gymnophorella n. gen. is also similar to some members of Cymuloporidae Winston and Vieira, Reference Winston and Vieira2013, Electridae d'Orbigny, 1852 (e.g., Pyripora magna Larwood, Reference Larwood and Larwood1973), and Calloporidae Norman, Reference Norman1903 (e.g., Tylopora Lang, Reference Lang1917) in having oligoserial colonies, and in the morphology of the autozooids showing a well-developed gymnocyst. It differs in having deep opesiular indentations outlining a tongue-like process medially. In addition, Cymuloporidae have colonies exclusively uniserial, and opesia occupying the distal half of the zooid total length; P. magna has a reduced cryptocyst and larger opesiae; and Tylopora spp. has ovicells, avicularia, and sometimes spines on the cryptocyst.

Figure 7. Steginoporella cornuta (Osburn, Reference Osburn1950), Recent, Acapulco, Mexico, Pacific Ocean (holotype SBMNH 635758) (photos courtesy of V. Delnavaz): (1) detail of the autozooids, note the absence of gymnocyst, (2) detail of a B-zooid; Recent, Isla Rancheria, Panama, Pacific Ocean (paratype SBMNH 636427): (3) group of autozooids with reduced gymnocyst in the proximal area, kenozooids (white arrow) and B-zooid (black arrow), (4) autozooid with uncalcified polypide tube (white arrow) and B-zooid (black arrow). Gymnophorella hadra n. gen. n. sp. from the Siamaná Formation, Arroyo Uitpa (paratype MUN-STRI-47643): (5) general view of the uni- and biserial stages, note the extended, proximal gymnocyst. Scale bars are (1) 0.5 mm; (2) 0.25 mm; (3–5) 1 mm.
Although the paratype of the Recent Pacific species “Steginoporella cornuta” (SBMNH 636427) exhibits some morphological differences compared to the holotype, and it is likely a new species, its description is not within the objectives of this paper.
Gymnophorella hadra new species
Figures 7.5, 8; Table 11

Figure 8. Gymnophorella hadra n. gen. n. sp. from the Siamaná Formation, Arroyo Uitpa (holotype MUN-STRI-47640): (1) detail of the regular autozooids, (2) detail of two autozooids with intramural buds and opesia showing opesiular indentations; (paratype MUN-STRI-47641): (3, 4) general view of the multiserial stage, (5) close-up of autozooids and a kenozooid (arrowed). Scale bars are (1, 4) 0.5 mm; (2, 5) 0.25 mm; (3) 1 mm.
Holotype
MUN-STRI-47640. Paratypes: MUN-STRI-47641–47644, MUN-STRI-43526. From the early Miocene Siamaná Formation, Arroyo Uitpa, La Guajira, Colombia.
Table 11. Measurements of Gymnophorella hadra n. gen. n. sp. X = mean; R = observed range; SD = standard deviation; N = number of measurements.

Diagnosis
Colony encrusting, uni- to multiserial. Zooids oval, distinctly separated laterally by a narrow groove. Opesia terminal, trifoliate with deep opesiular indentations outlining a tongue-like process medially; distal opesial margin distinctly raised, lateral margin with two conspicuous tubercles. Cryptocyst granular, unperforated, slightly concave, occupying three-quarters of the zooidal length, surrounded by a raised oval rim that is constricted in the distal opercular region. Gymnocyst relatively extensive, smooth, slightly convex, surrounding the cryptocyst and the opesia, without edges between distal and proximal zooids, more extended proximally in early astogeny. B-zooids seemingly absent. Arrangement uni- and biserial in early astogeny. Kenozooids frequent in multiserial colonies.
Description
Colony encrusting, unilaminar, uni- to biserial in early astogeny and multiserial in the advanced stages. Bifurcation of the bi- and triserial sections at angles of ~45°. Zooids oval to elliptical, separated laterally by a fine groove (mean L/W = 1.68). Opesia terminal, trifoliate, with large opesiular indentations proximally outlining a tongue-shaped process placed medially. Tubercles prominent, blunt, ovate to elliptic in cross-section, placed laterodistally on each side of the opesia. Cryptocyst extensive and granular, slightly concave, surrounded by a raised mural rim, commonly with intramural buds, occupying three-quarters of the zooid length and being distinctly constricted in the distal opercular region. Gymnocyst smooth, slightly convex, surrounding the cryptocyst and the opesia, extending continually between zooids of the same row except in some bifurcations, more extended in early astogeny and the proximal part of the zooid. Kenozooids frequent in the multiserial stages: triangular, elliptical, or similar in shape to the zooids. Polypide tube and rosette-plates not observed.
Etymology
From the Greek hadros, meaning well developed, declined in accordance to the gender of the genus, which is feminine, in reference to the well-developed gymnocyst differentiating this genus from other Steginoporellidae.
Remarks
The absence of B-zooids in the material found in the Siamaná Formation may be a bias related to the limited number of colonies available. For instance, in Steginoporella evelinae, the B-zooids are infrequent and similar in size and shape to the A-zooids (Winston et al., Reference Winston, Vieira and Woollacott2014). In the Siamaná Formation, Gymnophorella hadra n. gen. n. sp. was found growing over coral rubble of Porites sp., covered by coralline algae, and sharing the substrate with Antropora guajirensis n. sp., Cribrilaria sp., ?Hippopleurifera sp. 1, and Escharoides sp.
Family Poricellariidae Harmer, Reference Harmer1926
Genus Poricellaria d'Orbigny, Reference d'Orbigny1854
Type species
Poricellaria alata d'Orbigny, Reference d'Orbigny1854 from vicinity of Paris, France, Eocene; by original designation.
Poricellaria sp. indet.
Figure 9.1, 9.2; Table 12
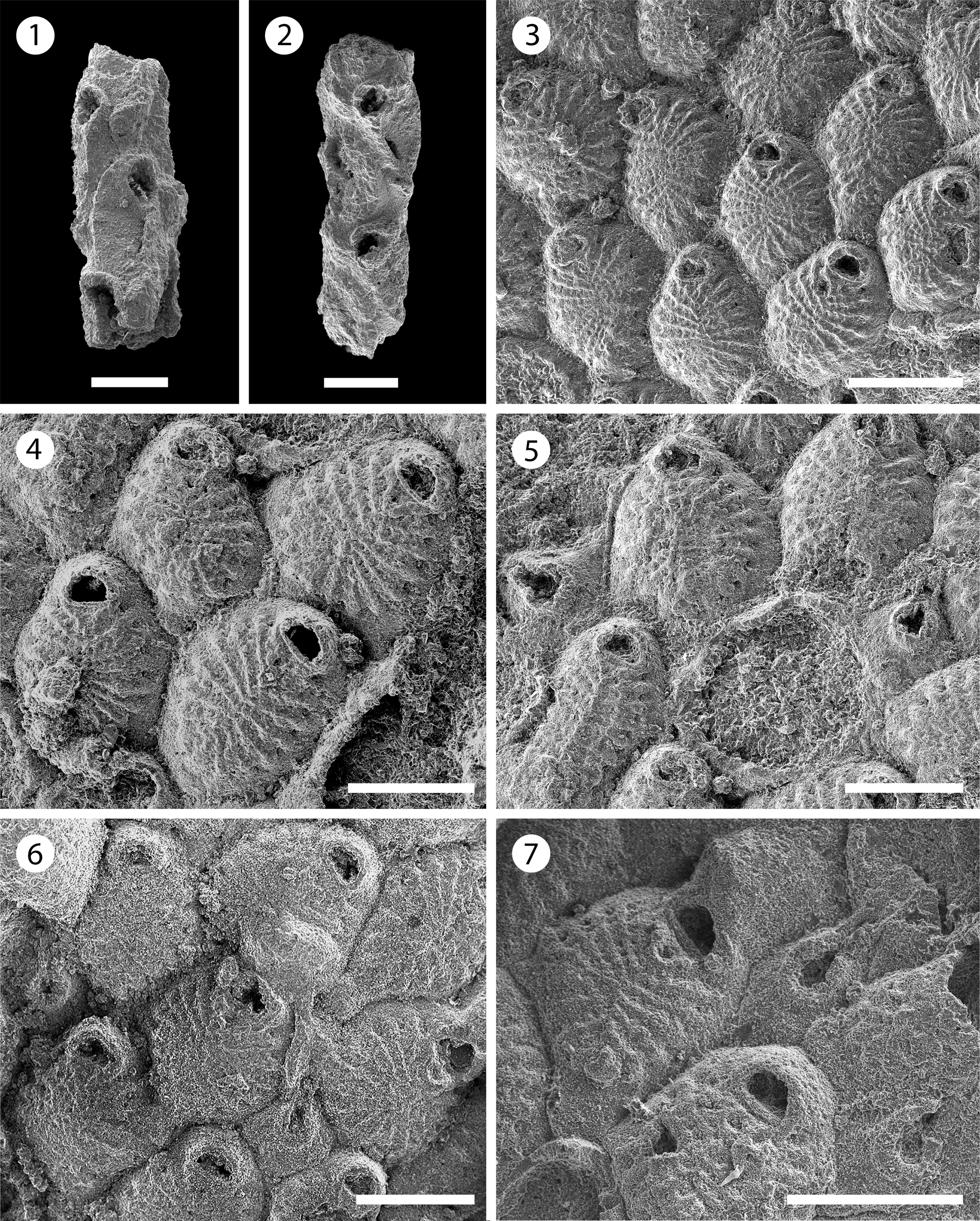
Figure 9. Poricellaria sp. indet. (MUN-STRI-47645): (1, 2) branch fragments showing the oblique orifice and the slit-like opesiule on the cryptocyst. Cribrilaria multicostata n. sp. (holotype MUN-STRI-47650): (3) general view of the autozooids, (4) autozooids showing the orifice shape, spine bases, and suboral lacuna, (5) detail of the avicularium with flared rostrum; (paratype MUN-STRI-47651): (6) fertile zooid with type A ovicell; (paratype MUN-STRI-47652): (7) detail of the avicularium and ovicell. Scale bars are (1) 0.15 mm; (2, 4–6) 0.2 mm; (3, 7) 0.25 mm.
Table 12. Measurements of Poricellaria sp. indet. X = mean; R = observed range; SD = standard deviation; N = number of measurements.

Occurrence
Early Miocene, Siamaná Formation, Arroyo Ekieps, Colombia.
Description
Colony erect. Branches slender and sub-circular in cross-section. Zooids pyriform (mean L/W = 1.80), curved distally, arranged in ?three longitudinal, alternated rows. Orifice terminal, semi-circular, oblique, tilted at an angle of 50–60° relative to the branch axis, facing frontally and laterally, borders raised. Cryptocyst well developed, oval, smooth, flat, with raised borders, perforated by a single slit-like opesiule, lying adjacent to the raised edge. Gymnocyst well developed, smooth, covering the proximal area. Avicularia adventitious, small, placed on the proximal gymnocyst of each autozooid, rostrum acute and rounded, proximolaterally directed, pivotal bar absent or not preserved. Ovicells not observed.
Remarks
Only two broken and recrystallized fragments were found in the samples. However, the inclined zooids with the oblique opesia and slit-like opesiule, as well as the avicularia placed on the gymnocyst of each autozooid, are features diagnostic of the genus Poricellaria (Harmer, Reference Harmer1926, p. 313). Nine species of this genus are known worldwide; among these, only Poricellaria vernoni Cheetham, Reference Cheetham1963 is known from North America. This species from the Oligocene of Florida differs from Poricellaria sp. indet. in having a finely perforate cryptocyst. Cheetham (Reference Cheetham and Larwood1973) found a specimen of Poricellaria in the Caribbean Miocene, which he identified as P. aff. ratoniensis; subsequently, Cheetham et al. (Reference Cheetham, Sanner and Jackson1999) reported it as Poricellaria new species 1 (figured in NMiTA Database, 1996–2016) from the Miocene (ca. 15.7–3.9 Ma) of the Dominican Republic. Although the Siamaná material resembles the species found by Cheetham et al. (Reference Cheetham, Sanner and Jackson1999), even in the size of the zooids, the poor preservation and scarcity of specimens preclude any further comparison and the description of a new species. In the Siamaná Formation, Poricellaria sp. indet. was found in the sediment attached to the coral Goniopora hilli, co-occurring with the bryozoan Nellia cf. N. tenella, as is common in other fossil localities and environments (Winston and Cheetham, Reference Winston, Cheetham, Eldredge and Stanley1984), as well as Margaretta sp., Ditaxiporina sp., and Mecynoecia sp.
Superfamily Cribrilinoidea Hincks, Reference Hincks1879
Family Cribrilinidae Hincks, Reference Hincks1879
Genus Cribrilaria Canu and Bassler, Reference Canu and Bassler1929
Type species
Eschara radiata Moll, Reference Moll1803 from the Mediterranean Sea, Recent; by original designation.
Cribrilaria multicostata new species
Figure 9.3–9.7; Table 13
Holotype
MUN-STRI-47650. Paratypes: MUN-STRI-47651–47656. From the early Miocene Siamaná Formation, Arroyo Ekieps, La Guajira, Colombia.
Table 13. Measurements of Cribrilaria multicostata n. sp. X = mean; R = observed range; SD = standard deviation; N = number of measurements.

Diagnosis
Colony encrusting, unilaminar. Zooids oval to rhomboidal. Orifice D-shaped, bearing five spine bases. Lacuna broad, placed in a triangular area between the orifice and the first pair of costae. Frontal shield formed by 13–17 slender costae. Gymnocyst narrow, surrounding the costate shield and the orifice. Interzooidal avicularia with flared rostrum, and rhombic cystid. Ooecium hyperstomial, subglobular, produced by the distal zooid (type A of Bishop and Househam, Reference Bishop and Househam1987), smooth, imperforate, and with a median umbo.
Description
Colony unilaminar, forming extensive and irregular encrustations. Zooids oval to rhomboidal, distinctly separated by deep grooves, longer than wide (mean L/W = 1.17). Orifice transversely D-shaped, bearing five spine bases in non-ovicellate zooids and four in ovicellate zooids, proximal border thick and straight. Frontal shield extensive and convex, formed by 13–17 slender costae; first pair of costae forming a ‘V’-area with a large lacuna visible in some zooids, obliterated by sediment in others; five to six intercostal pores. Gymnocyst narrow. Interzooidal avicularia frequent, large, placed on a rhombic cystid with a well-developed gymnocyst; rostrum flared at the tip with slightly narrow middle portion, oriented distally. Ovicell type A of Bishop and Househam (Reference Bishop and Househam1987), wider than long; ooecium surface smooth and with a median umbo or keel, not punctate.
Etymology
From the Latin multus, meaning many, and costa, meaning rib in reference to the greater number of costae that distinguish this species from other Cribrilaria spp. with flared avicularia, plus the suffix -ata, pertaining to.
Remarks
Two fossil species of Cribrilaria from North America, both from the early Oligocene, are known: Cribrilaria anaticula Canu and Bassler, Reference Canu and Bassler1920 and Cribrilaria carolinensis Gabb and Horn, Reference Gabb and Horn1862. They resemble Cribrilaria multicostata n. sp. in having five oral spines and large interzooidal avicularia. However, C. multicostata differs from both in having avicularia with a flared rostrum. Nine Recent Cribrilaria morphospecies are described in the literature having avicularia with flared mandibles (Harmelin, Reference Harmelin2006), seven of them at species level: C. flabellifera (Kirkpatrick, Reference Kirkpatrick1888) and C. vaceleti (Harmelin, Reference Harmelin2006) from the Indo-Pacific; C. arrecta Bishop and Househam, Reference Bishop and Househam1987, C. atlantis (Harmelin, Reference Harmelin2006), C. macaronensis (Harmelin, Reference Harmelin2006), and C. mikelae (Harmelin, Reference Harmelin2006) from the central Atlantic and Mediterranean Sea; and C. smitti (Winston, Reference Winston2005) from Florida and the Caribbean. Cribrilaria multicostata n. sp. is distinguishable from all of these by having a greater number of costae, up to 17 instead of 5–12, and five oral spines instead of six or seven (except in C. mikelae, which also bears five). Cribrilaria smitti, the geographically closest congener, differs also in having the first pair of costae more pronounced, leaving a wider space between it and the orifice, where it develops a bifid median umbo. In the Siamaná Formation, Cribrilaria multicostata n. sp. was found encrusting the surface of the coral Acropora panamensis, co-occurring with the bryozoans Cribrilaria nixor n. sp., Poricella sp., Hippoporina sp., and ?Hippopleurifera sp. 2.
Holotype
MUN-STRI-47657. Paratype: MUN-STRI-47658. From the early Miocene Siamaná Formation, Arroyo Ekieps, La Guajira, Colombia.

Figure 10. Cribrilaria nixor n. sp. (holotype MUN-STRI-47657): (1–3) autozooids (showing the orifice shape, spine bases, and suboral lacuna) and interzooidal avicularia, (4) fertile zooid with type B ovicell. All specimens are from the Siamaná Formation, Arroyo Ekieps locality. Scale bars are (1–3) 0.3 mm; (4) 0.25 mm.
Table 14. Measurements of Cribrilaria nixor n. sp. X = mean; R = observed range; SD = standard deviation; N = number of measurements.
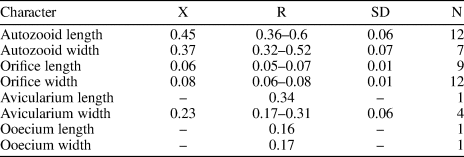
Diagnosis
Colony encrusting. Zooids oval to elliptical. Orifice D-shaped, bearing five spine bases. Lacuna broad and elliptical. Frontal shield formed by 17–22 costae. Gymnocyst narrow. Interzooidal avicularia with slender rostrum, cystid rhombic with extensive gymnocyst. Ovicell hyperstomial, ooecium produced by the distal zooid or kenozooid (type A and B, Bishop and Househam, Reference Bishop and Househam1987), smooth, imperforate, and with a median keel.
Description
Colony encrusting, multiserial, unilaminar. Zooids ovate to elliptical, distinctly separated by shallow grooves (mean L/W = 1.21). Orifice semicircular, wider than long; proximal border straight, formed by a thin bar. Five oral spine bases in non-ovicellate zooids and four in ovicellate zooids. Frontal shield extensive, slightly convex to flat, formed by 17–22 slender costae, tapering at the distal end, and fusing in the middle line. Costae separated by two to five circular intercostal pores. Suboral lacuna broad, elliptic, flanked by the first pair of costae forming a ‘V’. Gymnocyst very narrow. Interzooidal avicularia frequent, rostrum long and slender, cystid rhombic with a well-developed gymnocyst, oriented distally, sometimes lying on the frontal shield of the distal zooid, opesia oval. Ooecium type A and B (Bishop and Househam, Reference Bishop and Househam1987), longer than wide, surface smooth, not punctate, and with a median keel. Kenozooids present.
Etymology
From the Latin nixor, meaning leaning against, and used as a name in apposition, in reference to the interzooidal avicularia that lean against the frontal shield of the distal autozooids.
Remarks
Although a single colony is available for this species, several features (e.g., the number of costae, length of the zooids, type of ovicell budding from a kenozooid [type B], wider avicularian opesia, and long and slender rostrum) allow Cribrilaria nixor n. sp. to be distinguished from Cribrilaria multicostata n. sp. occurring in the same deposits. Cribrilaria nixor n. sp. differs from Cribrilaria anaticula and Cribrilaria carolinensis, both recorded in the early Oligocene of North America (Canu and Bassler, Reference Canu and Bassler1920, p. 297), in having a greater number of costae (17–22 vs. 10–12) and in the shape of the avicularian rostrum, which is duck beak-shaped in C. anaticula, or thin and channeled in C. carolinensis. In the Siamaná Formation, Cribrilaria nixor n. sp. was found encrusting the surface of the coral Acropora panamensis, co-occurring with the bryozoans C. multicostata n. sp., Poricella sp., Hippoporina sp., and ?Hippopleurifera sp. 2.
Genus Figularia Jullien, Reference Jullien1886
Type species
Lepralia figularis Johnston, Reference Johnston1847 from Cornwall, United Kingdom, Recent; by original designation.
Figularia bragai new species
Figure 11.1–11.5; Table 15
Holotype
MUN-STRI-47647. Paratypes: MUN-STRI-47648, MUN-STRI-47649. From the early Miocene Siamaná Formation, Arroyo Ekieps, La Guajira, Colombia.
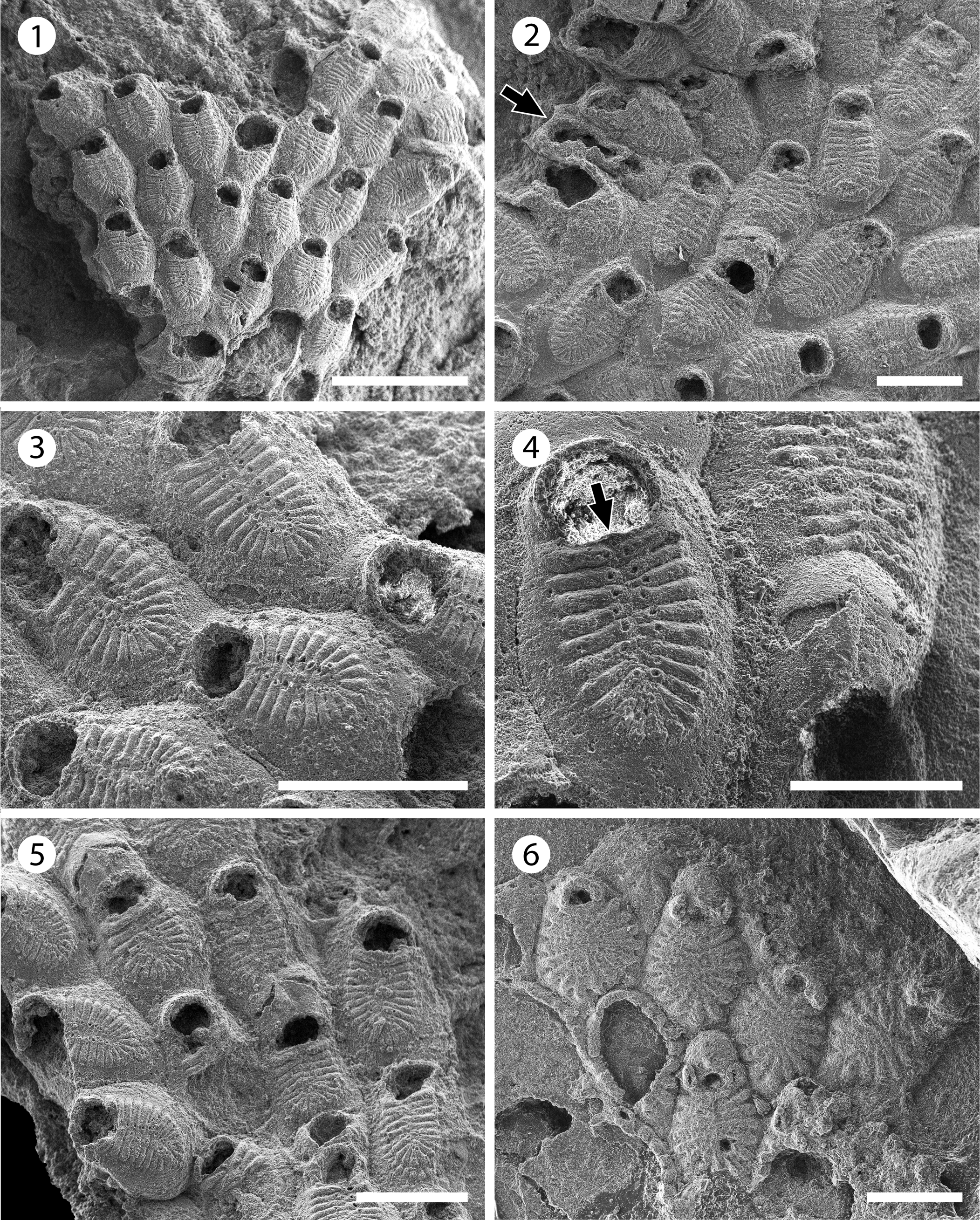
Figure 11. Figularia bragai n. sp. (MUN-STRI-47647): (1) general view of the colony, (2) autozooids and interzooidal avicularia (arrowed), (3) close-up of autozooids showing the frontal shield (costae and pelma) and the smooth gymnocyst, (4) close-up of a zooid showing the shape of the orifice, the suboral pore (arrowed), and an ovicell, (5) group of ovicellate and non-ovicellate zooids. Glabrilaria sp. indet. (MUN-STRI-47646): (6) general view of the colony. Scale bars are (1) 1 mm; (2, 3, 5) 0.5 mm; (4) 0.3 mm; (6) 0.25 mm.
Table 15. Measurements of Figularia bragai n. sp. X = mean; R = observed range; SD = standard deviation; N = number of measurements.

Diagnosis
Colony encrusting. Gymnocyst smooth, developed laterally and proximally. Frontal shield formed by 19–24 costae with a pelma located near the tip close to the median, fusion line of the costate shield. Orifice semi-circular with a centered, suboral pore. Putative, vicarious avicularia infrequent. Ooecium ovoidal with two drop-shaped or slit-like fenestrae, and a longitudinal suture medially.
Description
Colony encrusting, multiserial, uni- to multilaminar. Zooids large (mean L/W = 1.68), rhomboidal to elliptical, flat to slightly convex, distinctly separated by fine and shallow furrows. Gymnocyst smooth, slightly convex, developed laterally and proximally, occupying one-quarter of the frontal length of the zooid. Frontal shield oval, flat to slightly convex, a little raised above the level of the gymnocyst, formed by 19–24 adjacent costae, fused in the median line. Costae broad, bar-shaped in the central and distal area, tapering proximally, apparently separated by one to three slit-like intercostal lacunae; each costa bears a circular pelma placed close to the fusion line of the costae. Orifice terminal, wide, semi-circular, straight proximally, with a suboral, small, centered pore. Putative interzooidal avicularia infrequent, narrow, almost half of the zooid width, but similar in length. Ovicell hyperstomial, ovoidal; ectooecium smooth, with a longitudinal suture in the median line; two drop-shaped or slit-like fenestrae, oriented transversally, exposing a smooth endooecium. Ancestrula unknown. Kenozooids absent.
Etymology
Named after Professor Juan Carlos Braga, paleontologist (Departamento de Estratigrafía y Paleontología, Universidad de Granada, Spain), in recognition of his support in the study of recent and fossil bryozoans from Colombia.
Remarks
Figularia bragai n. sp. resembles the Miocene species from Indonesia ?Filaguria kalimantanensis Di Martino and Taylor, Reference Di Martino and Taylor2015 in the size of zooids, number of costae, position of the pelma, and the morphology of the ooecium. However, the latter species has putative vicarious avicularia similar to autozooids, and two lateral oral spine bases, which are diagnostic of the genus Filaguria Moyano, Reference Moyano1991 and absent in the Colombian material. However, in Figularia bragai n. sp. we observed a single polymorph, which can be interpreted either as a putative vicarious avicularium with spoon-shaped rostrum and complete cross-bar, or as a malformed autozooid with broken costate shield, constricted between two neighboring zooids with reversed polarity.
Vicarious avicularia with a spoon-shaped rostrum have been described for several species of Figularia: the Recent species F. fissa (Hincks, Reference Hincks1880) and F. fissurata Canu and Bassler, Reference Canu and Bassler1929, and Miocene species F. rhodanica Li, Reference Li1990 (see Li, Reference Li1990, pl. 2, fig. 11), but they all differ from the new species in the number of costae, number and size of pelmata, and/or characters of the ooecium (Rosso et al., Reference Rosso, Di Martino and Ostrovsky2020). Figularia bragai n. sp. also differs from the Recent species recorded from the Gulf of Mexico and listed as doubtful in Rosso et al. (Reference Rosso, Di Martino and Ostrovsky2020, table 4), ?Figularia ampla Canu and Bassler, Reference Canu and Bassler1928, and Figularia contraria Lagaaij, Reference Lagaaij1963, in the number of costae and size of the zooids and opesiae; the former species has six pairs of costae, and larger zooids (1.5 mm long, 1.0 mm wide); while the latter species has 8–11, more commonly nine, costae and a distinctly smaller opesia in ovicellate zooids. The absence of a pelma and proximal and lateral gymnocyst in the species described and figured by Canu and Bassler (Reference Canu and Bassler1920, p. 316, pl. 43, fig. 9) as ?Figularia crassicostulata (Canu and Bassler, Reference Canu and Bassler1920) from the late Eocene of North America suggests that this specimen does not belong to the genus Figularia (Rosso et al., Reference Rosso, Di Martino and Ostrovsky2020). In the Siamaná Formation, F. bragai n. sp. was found encrusting the underside of the coral Colpophyllia willoughbiensis as well as the lateral surface of Porites baracoaensis Vaughan, Reference Vaughan1919 and Acropora sp., sharing the substrate with an undetermined Cribrilinidae, ?Hippopleurifera sp. 2, Gemelliporidra sp., and ?Hippomenella sp.
Genus Glabrilaria Bishop and Househam, Reference Bishop and Househam1987
Type species
Puellina pedunculata Gautier, Reference Gautier1956 from Le Grand Congloué Island, Archipelago of Riou, France, Recent; by original designation.
Glabrilaria sp. indet.
Figure 11.6, 12; Table 15

Figure 12. Glabrilaria sp. indet. (MUN-STRI-47646): (1) close-up of a non-ovicellate autozooid, showing the orifice shape, oral spine bases, and pore chamber windows, (2, 3) close-up of ovicellate zooids with paired latero-oral avicularia, (4) detail of a kenozooid. All specimens are from the Siamaná Formation, Arroyo Ekieps locality. Scale bars are (1) 0.1 mm; (2, 3) 0.15 mm; (4) 0.25 mm.
Table 16. Measurements of Glabrilaria sp. indet. X = mean; R = observed range; SD = standard deviation; N = number of measurements.
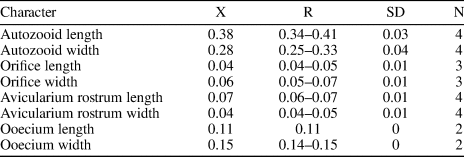
Occurrence
Early Miocene, Arroyo Ekieps, Siamaná Formation, Colombia.
Description
Colony encrusting, unilaminar. Zooids oval and small (mean L/W = 1.36). Orifice terminal, transversely D-shaped, bearing six to seven spine bases in non-ovicellate zooids. Gymnocyst very narrow, developed proximally and laterally. Frontal shield formed by 13–15 costal ridges, fused in the middle line without forming a median suture or umbo. Between the costae, five or six lacunae are visible. A pair of small adventitious avicularia with a triangular rostrum located on each side of the orifice of ovicellate zooids, oriented distally; rostrum small, raised at its distal end. Ooecium globular, produced by the distal kenozooid, apparently with a proximo-median umbo or keel. Kenozooids present. Ancestrula not preserved.
Remarks
Ten species of Glabrilaria have been recognized to date. Among them, the Mediterranean Glabrilaria pedunculata (Gautier, Reference Gautier1956) has the oldest record, ranging from the late Miocene (Pouyet, Reference Pouyet2000) to the Recent. In the American continent, two Recent species, Glabrilaria hirsuta Rosso in Rosso et al., Reference Rosso, Beuck, Vertino, Sanfilippo and Freiwald2018 and Glabrilaria polita Rosso in Rosso et al., Reference Rosso, Beuck, Vertino, Sanfilippo and Freiwald2018, have been recorded in the deep waters of the central West Atlantic, associated with coral fragments (Rosso et al., Reference Rosso, Beuck, Vertino, Sanfilippo and Freiwald2018). The Siamaná species differs from G. pedunculata and G. polita in having 6–7 (and not five) oral spine bases in non-ovicellate zooids. Although G. hirsuta also has seven oral spines, it differs from the present material in having on average a greater number of costae (13–15 vs. 14–18) and a larger ooecium. However, the Siamaná colony is too small and poorly preserved to observe the characteristic spiny ornamentation, the transversal processes of the ooecia, and/or the clustering of kenozooids typical of G. hirsuta (Rosso et al., Reference Rosso, Beuck, Vertino, Sanfilippo and Freiwald2018). Owing to these reasons, we prefer to keep this species in open nomenclature. In the Siamaná Formation Glabrilaria sp. indet. was found encrusting a mollusk shell, sharing the substrate with Atoichos magnus n. gen. n. sp. and a poorly preserved cyclostome.
Discussion
The majority of the bryozoan species described here are cheilostomes (87%), among which the family Cribrilinidae, with four species, is the most diverse, as already observed in shallow- and deep-water ecosystems with carbonate substrates, both fossil and Recent (Winston, Reference Winston2016). The family Onychocellidae is represented by two species, while the remaining nine families are represented by only one species each. Only two species, both left in open nomenclature, are cyclostomes (13%).
Seven of the species (47%) found in the Siamaná Formation are new, including three cribrilinids (Cribrilaria multicostata n. sp., Cribrilaria nixor n. sp., and Figularia bragai n. sp.), an antroporid (Antropora guajirensis n. sp.), a microporid (Calpensia caribensis n. sp.), an onychocellid (Atoichos magnus n. gen. n. sp.), and a steginoporellid (Gymnophorella hadra n. gen. n. sp.).
The remaining species (53%) are left in open nomenclature owing to their poor preservation, the absence of diagnostic features as gonozooids or ovicells, or the scarcity of available material. Species with erect colonies were commonly found only as small branch fragments, while for those with encrusting colonies only a few autozooids were recorded.
In the assemblage, 73% of the species have encrusting colonies, 20% have erect articulated colonies, and the remaining 7% have erected rigid colonies. The dominance of encrusting forms is a typical pattern in coral reef environments, favored by the availability of hard substrates (Winston, Reference Winston1986; Hamdane and Moissette, Reference Hamdane, Moissette, Wyse-Jackson, Buttler and Spencer-Jones2002; Cuffey, Reference Cuffey and Hopley2011). By contrast, taxa with free-living colonies, which in the Caribbean region are diverse and abundant from the middle Miocene (from ca. 15 Ma) to the Recent (Cheetham and Jackson, Reference Cheetham, Jackson, Herrera-Cubilla and Jackson2000; Flórez et al., Reference Flórez, Montoya-Cadavid, Reyes-Forero and Santodomingo2007; O'Dea, Reference O'Dea2009) are absent in the material studied here. This is probably a sampling/environmental bias with the collection focused on the reef framework, whereas free-living bryozoans are adapted to live on the soft bottoms surrounding the reefs, as well as at greater depths in muddy environments (O'Dea, Reference O'Dea2009).
In the Siamaná Formation, encrusting bryozoans were found on the bases and undersurfaces of scleractinian corals (e.g., Alveopora tampae, Acropora panamensis, Colpophyllia willoughbiensis, Porites baracoaensis, Acropora sp., and Porites sp.), as well as on coralline algae that covered the coral rubble, and on mollusk shells. On the other hand, the erect articulated species were found in the sediment attached to the bases and inter-branch spaces of corals such as Goniopora hilli, A. tampae, and A. panamensis. Erect articulated bryozoans are also common in coral reefs (Winston, Reference Winston1986; Cuffey, Reference Cuffey and Hopley2011), both modern and fossil, commonly deeper inside the protected cavities (Hamdane and Moissette, Reference Hamdane, Moissette, Wyse-Jackson, Buttler and Spencer-Jones2002).
Most species were found at the locality Arroyo Ekieps (80%), whereas at Arroyo Uitpa only Antropora guajirensis n. sp., Smittipora sp. indet., and Gymnophorella hadra n. gen. n. sp. were recorded (20%). In both cases, the species were exclusive of the locality. The Arroyo Uitpa paleoenvironment was characterized by patch reefs developed in a shallow lagoon close to the shore-line, while Arroyo Ekieps was a barrier reef system (Flórez et al., Reference Flórez, Zapata-Ramírez and Klaus2019a, Reference Flórez, Zapata-Ramírez and Klausb; and unpublished data), which offered a wide variety of microhabitats enhanced by the structural complexity of the reef framework. This suggests an increase of bryozoan diversity with higher reef complexity, as has been observed in modern reefs off Andros and Eleuthera in the Bahamas (Cuffey and Fonda, Reference Cuffey and Fonda1977).
In the Siamaná Formation, cryptic encrusting bryozoan colonies contribute to the fill of cavities reinforcing the reef framework, and articulated colonies contribute to production of sediment, in accordance with the role of bryozoans in fossil and modern coral reef building in tropical shallow waters (Cuffey, Reference Cuffey1977, Reference Cuffey and Hopley2011; Insalaco, Reference Insalaco1998; Taylor and James, Reference Taylor and James2013; Ramalho et al., Reference Ramalho, Taylor, Moraes, Moura, Amado-Filho and Bastos2018).
Acknowledgments
Financial support for this study was provided by Colciencias (Colombia) across the scholarship Doctorados en el Exterior 728. We are grateful for the financial support of the expeditions to the project 7277 569 33195 (Colciencias), Ecopetrol S.A., STRI, University of Zurich, Universidad del Norte, NSF (Grant EAR 0957679), National Geographic Society, Anders Foundation, 1923 Fund, and G.D. and J. Walston Johnson. EDM has received funding from the European Research Council (ERC) under the European Union's Horizon 2020 research and innovation programme (grant agreement No 724324 to L.H. Liow). Special thanks to J.C. Braga and P.D. Taylor for the support and helpful comments on the manuscript, to P. Zapata for the collection of coral samples during the expedition 2011, and C. Jaramillo for encouraging the study. Thanks to V. Delnavaz (SBMNH) for SEM pictures of type material; I. Sánchez for assistance with SEM pictures; J. Souto, C.M. López-Fé, and L. Vieira for their taxonomic advice; R. Cuffey, P. Bock, and J. Cancino for sharing literature; ARES team and Wayúu community for help in field and logistics. B. Berning and K. Zágoršek provided useful comments that greatly improved the original submitted manuscript.
Appendix
Appendix 1. List of bryozoans found in the Siamaná Formation (Cocinetas Basin in La Guajira Peninsula, Colombia). The collection is hosted at the Mapuka Museum of the Universidad del Norte, Barranquilla-Colombia. Specimens studied but not included in the descriptions because poorly preserved are indicated with an asterisk (*).

































