Introduction
Mysidaceans are a group of shrimplike peracarid crustaceans. They are arrayed into three orders: Mysida, Lophogastrida, and Stygiomysida (Meland and Willassen, Reference Meland and Willassen2007; Wittmann et al., Reference Wittman, Ariani and Lagardère2014). The earliest record of mysidaceans is in the Triassic of France (Bill, Reference Bill1914) and China (Taylor et al., Reference Taylor, Schram and Shen2001). South China is famous for widespread exposures of marine deposits from Late Palaeozoic to Late Triassic, and the area is well known for fossil Lagerstätten with exceptional preservation of diverse fossils. Despite intensive excavations over decades, only a single mysidacean taxon, Schimperella acanthocercus Taylor, Schram, and Shen, Reference Taylor, Schram and Shen2001, has been reported from the Ladinian Stage of the Middle Triassic in South China. Here we report the discovery of a new mysidacean assemblage from the Anisian Stage of the Middle Triassic Luoping biota in SW China. This occurrence represents the oldest known assemblage of mysidaceans.
The Luoping biota is a typical conservation Lagerstätte with exceptional preservation. Mysidaceans are the most numerous animals within the Luoping biota (Hu et al., Reference Hu, Zhang, Chen, Zhou, Lü, Xie, Wen, Huang and Benton2011). More than ten thousand individuals have been recovered so far. Associated fossils include marine reptiles, fish, bivalves, gastropods, echinoderms, ammonoids, lingulid brachiopods, plants, and other arthropods. In addition to the mysidaceans, horseshoe crabs (Zhang et al., Reference Zhang, Zhou, Lu, Xie, Lou, Liu, Sun and Jiang2008), isopods (Fu et al., Reference Fu, Wilson, Jiang, Sun, Hao and Sun2010), lobsters (Feldmann et al., Reference Feldmann, Schweitzer, Hu, Zhang, Zhou, Xie, Huang and Wen2012), and shrimp (Schweitzer et al., Reference Schweitzer, Feldmann, Hu, Huang, Zhou, Zhang, We and Xie2014) have been described. Thylacocephalans and a single myriapod are currently under study. The depositional environment of the Luoping biota is interpreted as a semi-closed intraplatform basin, and the exceptional preservation of fossils is interpreted to have been due to anoxia of bottom water and microbial sealing (Hu et al., Reference Hu, Zhang, Chen, Zhou, Lü, Xie, Wen, Huang and Benton2011; Luo et al., Reference Luo, Chen, Hu, Zhang, Benton, Zhou, Wen and Huang2013).
The material studied here was recovered from the middle part of Member II of the Guanling Formation at two quarries: the Dawazi Quarry (Quarry 1 of Feldmann et al., Reference Feldmann, Schweitzer, Hu, Huang, Zhou, Zhang, Wen, Xie and Maguire2016 [2015]) and the Shangshikan Quarry (Quarry 2 of Feldmann et al., Reference Feldmann, Schweitzer, Hu, Huang, Zhou, Zhang, Wen, Xie and Maguire2016 [2015]), Luoping County, Yunnan Province, SW China (Fig. 1). The interval containing the fossil mysidaceans is approximately 16 m thick, and is primarily comprised of thinly laminated micritic limestone alternating with thin- to moderately thick-bedded silty limestone. Fossil mysidaceans were obtained by splitting finely laminated micritic limestone. Most individuals are preserved as carbonized specimens, compressed in dorsal or lateral views on the bedding surface. A few individuals bear intestines preserved in 3-D configuration. Although several hundred individuals were examined in the course of this study, 25 were selected as type specimens for inclusion in this work.

Figure 1 Map and collecting localities for mysidacean crustaceans.
In addition to describing the new species of lophogastrid mysidaceans representing the oldest known occurrence of the group, the presence of swarms of the organisms and the association of multiple species in single occurrences permits speculation on the lifestyle of the animals.
Materials
Repository and institutional abbreviation
Specimens in this study are deposited in the Invertebrate Paleontology Collection, Chengdu Institute of Geology and Mineral Resources, Chengdu, Sichuan Province, China, bearing the acronym LPI.
Systematic paleontology
Class Malacostraca Latreille, Reference Latreille1802
Order Lophogastrida Boas, Reference Boas1883
Family Eucopiidae Sars, Reference Sars1885
Diagnosis
Carapace large, last 2 or 3 thoracic somites exposed dorsally. Rostrum obtuse. Eyes normally developed. Antennal (antenna 2) scale with apical suture, outer margin smooth, without setae, with or without terminal spine. Labrum symmetric. Mandible; left mandible lacinia mobilis well developed, reduced, or absent; right mandible armed with fixed cusp in position of the lacinia mobilis; spine row absent, molar process well developed.
1st maxilliped exopod small, unarticulated. 2nd thoracopod developed as a gnathopod, exopod reduced. 3rd thoracopod forming a gnathopod. 4th thoracopod forming a gnathopod. 3rd–8th thoracopod endopods with distinct carpus and propodus, propodus unsegmented. Branchiae on thoracopods present. Marsupium composed of seven pairs of oostegites.
Pleonal pleural plates absent. 6th and 7th pleonal somites [pleomeres] fused. Female pleopods biramous. Uropod endopod setose around entire margin, statocyst absent; exopod with distal articulation [diaeresis], outer margin entire, terminating in distal spines. Telson apex entire to lateral margins constricted (adapted from Meland, Reference Meland2002; square brackets embrace emendations).
Remarks
Meland (Reference Meland2002) published an illustrated, interactive key to eight extant families included within order Mysidacea. The key is based upon a broad spectrum of characters and is illustrated to clarify terminology. Among these, features of the antennae, carapace, pleon, telson, and appendages are preserved on specimens from the Luoping Biota. In fact, a sufficient number of features are present that specimens can be placed within families with a high degree of confidence. This plexus of characters is entirely compatible with placement within Eucopiidae and excludes placement in other morphologically similar families. Thus, Eucopiidae now includes three fossil genera: Eucopia Dana, Reference Dana1852; Schimperella Bill, Reference Bill1914; and Yunnanocopia new genus.
Three families of the eight considered by Meland (Reference Meland2002) to be united within Mysidacea keyed out as most similar morphologically: Eucopiidae, Lophogastridae Sars, Reference Sars1870, and Mysidae Haworth, Reference Haworth1825. Lophogastridae is characterized by a plate-like rostrum and an antennal scale with a serrated outer margin, as seen in the fossils. Some subfamilies of Mysidae also key out close to Eucopiidae. The marsupium of Mysinae Haworth, Reference Haworth1825, within Mysidae, is composed of only two pairs of oostegites, whereas that in the present specimens has at least six, and probably seven pairs. Similarly, another mysid subfamily, Mysidellinae Norman, Reference Norman1892, is characterized by an elongate rostrum, a marsupium with three pairs of oostegites, a cleft telson, and an exopod lacking a diaeresis, none of which are seen in the new specimens. Thus, Eucopiidae is the only family that can accommodate the Luoping specimens.
Meland (Reference Meland2002) considered Mysidacea as an order subdivided into families but without subordinal arrangement. Hessler (Reference Hessler1969), among others, also considered Mysidacea to be of ordinal rank, but subdivided the order into two suborders: Mysida Boas, Reference Boas1883, and Lophogastrida Boas, Reference Boas1883, including Eucopiidae.
As an alternative to the higher level classifications of Hessler (Reference Hessler1969), Meland (Reference Meland2002), and others, other higher level classifications of eucopiids and related forms have elevated Lophogastrida and Mysida to the level of order (Schram, Reference Schram1986; Martin and Davis, Reference Martin and Davis2001; Meland and Willassen, Reference Meland and Willassen2007; Wittmann et al., Reference Wittman, Ariani and Lagardère2014; Meland et al., Reference Meland, Mees, Porter and Wittmann2015); however, the concept of Eucopiidae is essentially unchanged. The diagnosis of Eucopiidae presented by Wittmann et al. (Reference Wittman, Ariani and Lagardère2014) generally conforms to that of Meland (Reference Meland2002), with Eucopiidae as a family within Lophogastrida instead of Mysidacea. The nature of preservation of the Luoping material is such that it is not possible to assess the higher-level ranks of Mysidacea sensu lato in any greater detail. Therefore, we follow the most recent classification placing Eucopiidae within order Lophogastrida. We retain the diagnosis of Meland (Reference Meland2002) for Eucopiidae because it permits clear placement of fossil material within the family, utilizing features preserved on fossil specimens, which lack many morphological features commonly used in other biological classifications.
Yunnanocopia new genus
Type species
Yunnanocopia grandis new species, by original designation.
Other species
Yunnanocopia longicauda new species
Diagnosis
Antennal scale ovate to lanceolate with an acute tip; antennular segments are stout with first segment larger than second and third; precervical grooves inclined posterolaterally and do not cross midline; cervical groove concave forward; two or three thoracic segments exposed dorsally; pleonal pleurae strongly reduced or absent; telson and uropods lack any indication of having been setose.
Etymology
The generic name refers to Yunnan, the province from which the specimens were collected, and the Latin copia=abundance, in reference to the abundance of specimens referable to the genus. The gender is feminine.
Remarks
The specimens collected from the Luoping Biota represent taxa that can confidently be assigned to a new genus. Previously, Taylor et al. (Reference Taylor, Schram and Shen2001) described Schimperella acanthocercus from Triassic rocks in Guizhou, China. Species of Schimperella differ in several ways from the Luoping material sufficiently to warrant description of a new genus. Schimperella Bill, Reference Bill1914, was based upon two species from the Triassic of France: S. beneckei, the type species by subsequent designation of Hessler (Reference Hessler1969), and S. kessleri. The unique nature of S. kessleri has been questioned (Taylor et al., Reference Taylor, Schram and Shen2001). Certainly the illustrations of the species (Bill, Reference Bill1914, pl. 16, figs. 3, 4) suggest that it may be the female counterpart of S. beneckei. In addition, Schimperella spp. have been reported from the Middle Triassic Meride Limestone in northeastern Italy (Largi and Tintori, Reference Larghi and Tintori2007) and from the Middle Triassic (middle Anisian) Strelovec Formation in Slovenia (Križna and Hitij, Reference Križnar and Hitij2010). This latter occurrence might be shown to be more or less contemporaneous with the Luoping occurrences; however, that has yet to be tested.
Schimperella kessleri exhibits a well-developed marsupium, whereas the type species appears to be a male. Regardless, species of Schimperella are characterized by an extremely large, obovate antennal scale; slender basal antennular segments; a weak, transverse precervical groove; transverse cervical groove; carapace covering nearly all thoracic segments dorsally; pleonal pleura that are moderately well developed; and setose telson and uropods. By contrast, the antennal scale of Yunnanocopia spp. is ovate with an acute tip, the antennular segments are stout, the precervical grooves are inclined posterolaterally, the cervical groove is concave forward, two or three thoracic segments are exposed dorsally, pleonal pleurae are strongly reduced or absent, and the telson and uropods lack any indication of having been setose. Thus, assignment of the lophogastrids from the Luoping Biota to a new genus seems fully warranted.
Yunnanocopia grandis new species
Figures 2.1–2.7, 3.1–3.6, 4.1–4.6

Figure 2 Yunnanocopia grandis new genus new species, LPI-41683, paratype: (1) entire animal; (2) anterior of body, S=antennal scale; (3) thoracic somites, C=cervical groove, Ap=apodemes of thoracic somites; (4) posterior end of thorax, Co=coxae of thoracic appendages; (5) closeup of pleonal somites; (6) pleon including telson and uropods; (7) pleonal somite 6, telson, and uropods. Scale bar 1=1 cm; scale bar 6=2 mm, remaining scale bars=1 mm.

Figure 3 Yunnanocopia grandis new genus new species, LPI-40749, holotype: (1) entire animal; (2) anterior view, A1=antennules, A2=antennae; (3) thorax, PC=pre-cervical groove, C=cervical groove, p=posterior margin of carapace, note at least 2 thoracic somites not covered by carapace; (4) posterior thorax and pleon, T=thoracic somites not covered by carapace; (5) pleonal somites with pleopods (Pl); (6) Telson (T) and uropods showing diaeresis (D) on exopod. Scale bar 1=1 cm; remainder=1 mm.

Figure 4 Yunnanocopia grandis new genus new species: (1-2) LPI-33341, paratype, (1) anterior view of thorax, cervical groove, and basal elements of antennal scale, (2) thorax with oostegites (O); (3-6) LPI-41689, paratype, (3) anterior view showing cervical groove and antennal scales; (4) thorax and anterior pleon; (5) pleon with basal elements of pleopods; (6) telson and uropods. Scale bars=1 mm.
Diagnosis
Third element of antennular base shorter than second; antennal scale weakly convex laterally, ovoid, longer than wide; endophragmal skeleton narrows posteriorly; at least six pairs of oostegites form prominent marsupium; pleonal tergites irregularly nodose; pleura not evident; telson spatulate; uropods long, flabellate.
Description
Large, stout eucopiids. Antennules biflagellate; antennular base with first element longer than wide, rectangular, outer and inner margins weakly convex; second segment short, about as wide as long, rectangular; third segment shorter, wider than long, narrowing axially, extends nearly to tip of antennal scale. Antennular flagellae of equal diameter, but two different lengths; inner one is much shorter, directed straight forward. Antenna longer than the antennules. First antennal segment longer than wide, rectangular; second segment apparently rectangular, about as wide as first segment but shorter; flagellum more stout than antennular ones. Antennal scale weakly convex laterally, ovoid, longer than wide; thickened rim on outer margin. Basal element of antennal scale equidimensional, outer margin weakly convex, inner margin apparently broken, distal margin sinuous, with a small spine on outer corner.
Cephalothorax longer than wide, anterior margin obscured; weakly convex laterally; deeply concave posteriorly, rimmed, posterolateral corner projected as rounded, posterolaterally directed flange; rostrum inferred to be short, triangular. Cervical groove rims anterolateral margin and curves from anterolateral margin posteriorly at 45° angle, then extends straight to axis at much lower angle to midline; as it approaches midline, it curves and parallels midline for short distance before crossing midline. Precervical groove intersects cervical groove laterally, then arcs concave forward and traverses obliquely about one-quarter the distance toward axis, defining hepatic region; expressed as grooves on the holotype. Cephalic region short. No evidence of lateral rim or any ornamentation posterior to cervical groove. Two or possibly three thoracomeres not covered by carapace dorsally (40749). Underside of anterior part of cephalothorax rimmed.
Endophragmal skeleton well calcified, six apodemes visible, straight, inclined posterolaterally from midline; seven pairs of coxal holes evident, all posterior to cervical groove; endophragmal skeleton narrows posteriorly, widest at position of third and fourth coxal holes.
Marsupium well developed on female specimens. Right oostegites ovate, posterior margin concave forward, with thickened rim, posterior-most oöstegite apparently arising on last thoracomere, extending from position of basal elements of thoracopods over half the distance across preserved portion of animal; six are preserved. Left oostegites with convex-forward, thickened anterior margin. Left oostegites overlap the right ones.
Six pleonites, first five about equal length, width decreases slightly posteriorly; sixth pleonite longer than the fifth; tergites irregularly nodose, rimmed along posterior margin of each pleonite, lateral margins rounded, bordered by thickened rim. In ventral aspect pleonites are strongly calcified anteriorly and axially, flaring anteriorly into sharp processes. Pleopods visible on pleonites 3–6, flagellate, multiarticulate, nearly as long as somite width; basal element ovate, slightly longer than wide.
Telson spatulate, tapers slightly posteriorly, anteriorly not quite as wide as sixth pleonal somite, axially sulcate from anterior end nearly to termination dorsally and with broad granular ridge arising proximally, bifurcating distally, on ventral side; weakly rimmed marginally; distally smoothly rounded, lateral margins of telson appear granular.
Uropods long, flabellate, exopod markedly longer than endopod, diaeresis of exopod lies posterior to end of telson, two nodes at about midwidth of diaeresis (40749). Endopod flabellate, extending to tip of telson.
Etymology
The trivial name refers to the relatively large size of specimens referred to this species.
Types
Holotype, LPI-40749, and paratypes LPI-41683, 41684, 41648, 41689, 33341, and 41390.
Remarks
Specimens referred to Yunnanocopia grandis are preserved in both dorsal and ventral aspect. The anterior appendages and the cephalic region tend to be well represented, and the thoracic region is poorly exposed or absent axially, but well represented laterally. Pre-cervical and cervical grooves are well impressed, and the pleon and tail elements are well preserved. Several specimens exhibit parts of the endophragmal skeleton that bears a close resemblance to that seen in Schimperella acanthocercus, consisting of biconcave forward rays emanating from the midline. However, the rays are somewhat thicker on S. acanthocercus. Pleonal sternites on both species form triangular extensions from the axial region; the anterior and posterior rays of those of S. acanthocercus project axially at the same angle, whereas those of Y. grandis do not. The anterior ray lies in a nearly transverse position, and the posterior ray is directed posteriorly. Ventral views of the sternites of S. acanthocercus are not in evidence because those specimens tend to be preserved in dorsal or lateral aspect.
Yunnanocopia longicauda new species
Figures 5.1–5.6, 6.1–6.6, 7.1–7.6, 9

Figure 5 Yunnanocopia longicauda new genus new species: (1-3) LPI-41569, holotype, pleon, pleonites 1–6 labeled (Pl1–Pl6), note pleonite 6 much longer than others, telson (T) poorly preserved; (2) anterior view of well-preserved cephalic appendages, S=antennal scale, antennular basal segments 1, 2, 3, F=antennular flagellae, R=rostrum, E=eye; (3) fecal pellets associated with specimen, assumed to have been made by the same species; (4–6) LPI-41571, paratype, (4) posterior thorax and pleon showing trace of intestine; (5) anterior view of poorly preserved antennal scales and cervical groove (C); (6) telson (T) and uropods (U). Scale bars=1 mm.

Figure 6 Yunnanocopia longicauda new genus new species: (1, 4) LPI-41691, paratype, (1) entire animal and (4) anterior view showing stalked eye (E); (2) LPI-41573, paratype, entire animal showing diaeresis (D) on exopod of uropod; (3, 5, 6) LPI-41770, paratype, (3) entire animal; (5) pleon; (6) anterior view with antennal? flagellum (Fl) and thoracic somites (T). Scale bars=1 mm.

Figure 7 Yunnanocopia longicauda new genus new species: (1-2) LPI-41686, paratype, (1) entire animal, (2) pleon with intestinal trace (Int); (3) LPI-32393, paratype, most of animal with coxal bases marked (C); (4, 6) LPI-41696, paratype, (4) anterior view with stalked eye (E) and antennal scales; (6) posterior view of pleon and tailfan; (5) LPI 32549, paratype, lateral view of bases of thoracic appendages (P) and pleopods (Pl). Scale bars=1 mm.
Diagnosis
Long, slender eucopiids. Third article of antennular base longer than second; antennal scales lanceolate, sharp tipped; rostrum long, slender; endophragm narrow, apparently straight sided; pleonal tergites appear to be smooth; pleura strongly reduced; telson much longer than wide, margins convex, narrowing slightly posteriorly; uropods very long, slender.
Description
Antennular base with first segment longer than wide, rectangular, about two-thirds as wide as antennal scale; second article rectangular, wider than long; third article tapering distally, wider than long, longer than second article; antennular flagella stout. Antennal scales lanceolate, sharp tipped, thickened on smooth outer margin. Antennal flagellum(?) lies ventrally below antennal scale. In lateral view dorsal surface of antennal scale is arcuate, much longer than wide. There may be a diaeresis on the antennal scale. Specimen 41569 shows breaks on both sides that could be where diaeresis is positioned. Other specimens show what might be breaks, but 41770 does not show the diaeresis, so its presence is equivocal.
Rostrum(?) long. Precervical grooves extend anteroventrally and then curve posteroventrally. Cervical groove a concave-forward arc all the way across the carapace. Eyes on very short stalks, large; stalks about 0.33 mm long. Six or seven thoracomeres visible in ventral view. Six or seven apodemes visible. Apodemes are straight axially, then angle posterolaterally laterally. Six, maybe seven, coxal holes on left side. Entire endophragm narrow, apparently straight sided.
Coxal bases closely spaced, making entire endophragm quite narrow. At least six thoracic appendages poorly preserved, segmentation not visible, terminations not visible.
Pleonal somites rectangular; 1 through 3 about same length, 4 and 5 about equal in length; sixth pleonite longer than others. Pleonite 4 narrower than others. Intestinal tract well preserved, narrow, packed with globular pellets posteriorly; pleonal somites well preserved; terga appear to be smooth; pleura strongly reduced, rounded rectangular, weakly rimmed, arcuate groove or rim anteriorly on somites, convex forward, directed posteroventrally in lateral view. Pleopods on somites 3, 4, and 5 appear to have long, narrow elements proximally.
Telson much longer than wide, margins weakly convex, narrowing slightly posteriorly, widest about one-third distance posteriorly. Telson short compared to uropods. Uropods very long, slender, outer margin entire, with straight diaeresis. Uropodal exopod anterior and posterior to diaeresis longitudinally striate for entire length.
Rectangular (cylindrical) objects surrounding the specimen may be fecal pellets.
Etymology
The trivial name alludes to the extremely long, slender uropods.
Types
Holotype LPI-41569, and paratypes LPI-41686, 41571, 41770, 41573, 32333, 41694, 32549, and 41696.
Remarks
Three characters aligned these specimens to Eucopiidae: an antennal scale with a smooth outer margin, normal-sized eyes, and uropods with a smooth outer surface. Beyond those characters, Yunnanocopia longicauda also bears some resemblance to its congener, Y. grandis, so that placement in the same genus is warranted. The two species share a lanceolate to ovate antennal scale with acute tip, an antennular base with the first segment larger than the others, a precervical groove that does not cross the midline, a concave-forward cervical groove, strongly reduced to absent pleonal pleura, and no indication of setae on the tail fan.
The relative size of elements two and three in the antennular base is different in the two species in that the third element is small and triangular in Y. grandis (Fig. 8.2), whereas that in Y. longicauda (Fig. 8.3) is approximately the same size as the second element and has a more rounded terminus. The antennal scale on Y. longicauda is broader and has a thickened outer margin, whereas the antennal scale is narrower and not obviously thickened on the margin of Y. grandis. The endophragm of Y. grandis is weakly convex laterally, whereas that of Y. longicauda is straight sided, corresponding to the overall shape of the organisms in that Y. grandis is more stout than the slender Y. longicauda. Ornamentation of nodes on the terga of Y. grandis contrasts with the smooth tergal surface of Y. longicauda. Reduced pleura are present on Y. longicauda, but are not evident on Y. grandis. Pleonite 6 is shorter on Y. grandis than on Y. longicauda, although that structure is longer than the other pleonites in each species (Fig. 8.2, 8.3). The telson of Y. grandis is long and spatulate and that of Y. longicauda is shorter and more rounded at the tip. The length of the uropods of the former is shorter than that of the latter.
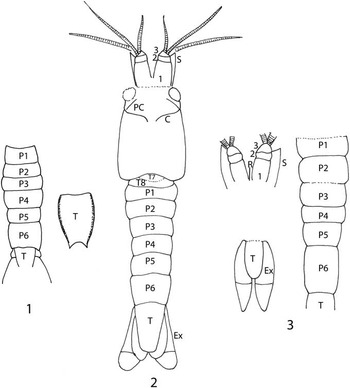
Figure 8 (1) Reconstruction of Eucopiidae species indeterminate. (2) Reconstruction of Yunnanocopia grandis new genus new species. (3) Reconstruction of Yunnanocopia longicauda new genus new species. C=cervical groove, Ex=exopod of uropod, PC=pre-cervical groove, P1–P6=pleonites 1–6, R=rostrum, S=antennal scale, T=telson, T7–T8=thoracic somites 7 and 8, 1–3=articles 1–3 of basal element of antennule. Drawings not to scale.
Clearly, the two forms are distinctly different morphologically. In addition, Y. grandis is less numerous and occurs as isolated individuals in the Luoping assemblage, whereas Y. longicauda is more numerous and occurs as swarms (Fig. 9). The presence of a rostrum on Y. longicauda is problematic. An elongate structure is present on LPI-41569, however it is displaced slightly from the longitudinal axis and its relationship to the carapace is not clear. The feature is called out (Fig. 5.2), but its identity must be questioned. No similar structure is evident on other specimens.
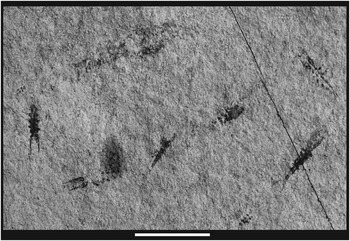
Figure 9 Swarm of Yunnanocopia longicauda new genus new species. Scale bar=1 cm.
The question remains to be resolved as to whether these differences warrant assignment to different genera or whether they can be united into a single genus. Examination of the form of the antennular base of three species of the extant Eucopia—E. australis Dana, Reference Dana1852, E. grimaldii Nouvel, Reference Nouvel1942, and E. linguicauda Tattersall, Reference Tattersall1955—demonstrates the third basal element is vastly different in the three species. That element on E. australis widens distally in concave arcs, and terminates in a broad, digitate margin. The third element on E. linguicauda is similar in having a digitate margin, but it forms the outer side of a triangular element. The third element of E. grimaldii is somewhat smaller, triangular, and the outer margin is undulose, rather than digitate. The shape of the carapace and the relative difference in length of pleonite 6 on the extant E. sculpticauda and E. unguiculata also differ markedly. Pleonite 6 is 1.6 times as long as pleonite 5 in E. sculpticauda, and 3.5 times as long as pleonite 5 in E. unguiculata. Pleonite 6 is 1.5 times as long as pleonite 5 in Yunnanocopia grandis and 2.2 times as long as pleonite 5 in Y. longicauda. The range in variation of morphology exhibited by Y. grandis and Y. longicauda compares well with that seen in extant species of Eucopia. Thus, we place the two species within a single genus until additional information warrants creation of a second genus.
Eucopiidae, species indeterminate
Figures 10.1–10.5, 11.1–11.5

Figure 10 Eucopiidae species indeterminate: (1) LPI-32802, swarm of specimens in typical occurrence for taxon; (2) LPI-32802.3, possible marsupium, o=oostegite; (3) LPI-32802.1, pleon, 1–6=pleonal somites, T=telson, U=uropod; (4) LPI-32802.2, pleon; (5) LPI-32802.4, posterior pleon and telson. Scale bar 1=5 mm; remainder=1 mm.

Figure 11 Eucopiidae species indeterminate: (1) 41335, entire animal showing short thorax and wide pleon; (2) 41335a, entire animal showing short thorax and wide pleon; (3) RMFCES3, pleon with well-preserved telson and uropods; (4) RMFCES4-1, pleonal somites and possible marsupium; (5) RMFCES4-2, pleon and well-preserved telson. Scale bars=1 mm.
Description
Small eucopiid. Carapace appears to be short relative to pleon, poorly preserved. Oostegites with arcuate, thickened anterior rims, disarticulated.
Pleon with six pleonites in ventral view; pleonite 1 is shorter than 2, 2 is shorter than 3, 3 slightly longer than 4, 4 and 5 same length, 6 appears longer than 5; Pleon nearly equal width for entire length. Width is 32% length excluding telson.
Apodemes weakly convex forward, becoming concave forward at lateral margins. Pleon is about twice as long as cephalothorax. Each somite is rimmed posteriorly.
Telson and uropods not well preserved. Telson seems to be longer than wide, quadrate, weakly convex lateral margins, with tiny, sharp spines at posterolateral corners. Uropods with broad, ovate basal segment, longer than wide. Endopod and exopod extend beyond tip of telson. Outer edge of exopod of uropod with thickened rim, may be serrate proximally. Uropods appear to have longitudinal striations.
Materials
LPI-32802 (part), 41335a, 42567, RMFCES1, RMFCES2, RMFCES 3, RMFCES4-1, RMFCES4-2, RMFCES4-3.
Remarks
Nine specimens examined in detail were used to frame the above description. The specimens are small and invariably incomplete, so that framing a description sufficient to erect an additional species is not prudent. The specimens do exhibit characters that distinguish them from Yunnanocopia grandis and Y. longicauda. The pleuron, which is longer than the poorly preserved carapace, has convex lateral margins, so that the widest part of the pleon is at pleonite 3. The telson is longer than wide, has convex lateral margins, and bears tiny spines on the posterolateral corners. The uropods are incompletely preserved only, suggesting that they are as long as, or longer than the telson. This combination of characters, particularly the outline of the pleuron and the morphology of the telson, are significantly different from the two named species. This species occurs in swarms of dozens of individuals (Fig. 10.1).
Reproduction
Taylor et al. (Reference Taylor, Schram and Shen2001) observed that little could be said about the reproductive strategy of Schimperella acanthocercus because there was no evidence of brooding structures in their specimens of that genus. However, examination of the illustrations of S. kessleri Bill, Reference Bill1914 (pl. 16, figs. 3, 4) clearly illustrate an extended marsupium with what appear to be seven oostegites (Fig. 12.1). No such structures are illustrated on S. beneckei suggesting that it was based upon a male and that S. kessleri was based upon a female. As originally suggested by Briggs et al. (Reference Briggs, Weedon and Whyte1993, p. 331), those specimens originally considered to represent two species should be assigned to a single species. By virtue of page priority, S. beneckei becomes the senior synonym and S. kessleri is its junior synonym.
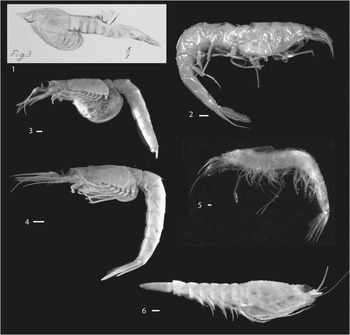
Figure 12 Mysidacea: (1) Lophogastrida, Eucopiidae, Schimperella kessleri, digital image of Bill (Reference Bill1914, pl. 16, fig. 3), showing probable marsupium of probable female; (2) Lophogastrida, Eucopiidae, Eucopia unguiculata, USNM 89756, lateral view, illustrating extremely thin, delicate cuticle; (3) Mysida, Mysidae, Mysis stenolepis, USNM 89733, female with well-developed marsupium; (4) Mysida, Mysidae, Mysis stenolepis, USNM 89732, female with marsupium tucked under thorax; (5) Lophogastrida, Eucopiidae, Eucopia major, USNM 283731, lateral view; (6) Lophogastrida, Lophogastridae, Lophogaster challengeri, USNM 235398, female with marsupium. Scale bars=1 mm.
Structures interpreted to be oostegites are present on specimens of Yunnanocopia grandis (Fig. 4.1, 4.2). Although specimens of extant, gravid Eucopia could not be located in the collections of the Smithsonian Institution, representatives of Mysis stenolepis Smith, Reference Smith1873, were available. One specimen, USNM 89733, exhibits a fully distended marsupium, in which eggs were visible (Fig. 12.3). Mysis Latreille, Reference Latreille1802, is the type genus of Mysidae, and that family is characterized by species bearing a reduced number of oostegites, generally three. Another M. stenolepis, USNM 89732, bore a marsupium, but it was tucked beneath the thorax (Fig. 12.4). Additionally, within the Lophogastrida, a specimen of Lophogaster challenger Fage, Reference Fage1942, USNM 235398, (Fig. 12.6) carries a marsupium bearing more than three oostegites, as would be anticipated in species within Eucopiidae.
Paleoecological implications
Extant lophogastrids are marine pelagic organisms that are largely confined to habitats below 200 m (Wittmann et al., Reference Wittman, Ariani and Lagardère2014). Studies summarized therein documented species of Eucopia Dana, Reference Dana1852, at depths greater than 2000 m. The fragile carapace and pleon of specimens of Eucopia spp. may suggest adaptation to great depth (Fig. 12.2, 12.5). Thus, although some Mysida occupy marine environments from tide pools to abyssal depths (Wittmann et al., Reference Wittman, Ariani and Lagardère2014, and references therein), the lophogastrids currently are not known to inhabit epipelagic settings. Although species of Yunnanocopia in all probability did not inhabit bathyal depths, it may be inferred that they did not occupy the surface plankton but, instead, swarmed over mid-depth to bottom-water areas. Taylor et al. (Reference Taylor, Schram and Shen2001, p. 310) referred to the mysidaceans, including lophogastrids, as preferring benthic habitats; but, current literature referred to above documents a pelagic lifestyle.
It is of note that Yunnanocopia is represented by two and possibly a third species. This is not unusual for eucopiids. Deep-water trawls in the mid-Atlantic frequently sample three species of Eucopia: E. grimaldii, E. sculpticauda, and E. unguiculata (personal communication, K. Meland, 2015). Thus, the discovery of two or three species of eucopiids in the Luoping Biota suggests that grouping of species is a behavior that has persisted since their early history.
Acknowledgments
Specimens forming the basis for the study were collected with the support of NSF OISE-1126137 to Feldmann and Schweitzer, National Geographic Society Grant 9128-12 to Feldmann, and China Geological Survey grants 121201114068001 and 1212011140051 to Hu and colleagues at Chengdu. Access to the spirit collections and facilitation of a loan of specimens for the research were provided by R. Lemaitre and K. Reed, United States National Museum, Smithsonian Support Center. Careful reviews by K. Meland, University of Bergen, Norway, and an anonymous reviewer substantially improved the manuscript. Associate Editor B. Pratt, University of Saskatchewan, also offered numerous constructive comments.














