Introduction
With 15 genera, Macraucheniidae Gervais, Reference Gervais1855 is one of the two more diverse and representative families of the order Litopterna, which is recorded from the late Eocene to Late Pleistocene–Early Holocene (Bond et al., 1999; Schmidt and Ferrero, Reference Schmidt and Ferrero2014). This order is part of the group called South American native ungulates (SANU) (Cifelli, Reference Cifelli1985; Bond, Reference Bond1999). Macraucheniidae includes middle- to large-sized herbivores and is considered a family more conservative in dental and postcranial aspects than Proterotheriidae because of its complete dentition without diastema and the presence of three digits (Paula-Couto, Reference Paula-Couto1979; Bond, Reference Bond1999). Moreover, the lineage of macraucheniids is characterized mainly by gradual retraction of the nasals and the backward shift of the nostrils, which reaches the maximum in Pleistocene taxa (Bond, Reference Bond1999; Cartelle, Reference Cartelle1999). Records of macraucheniids in the Pleistocene are widely distributed throughout South America. In the Pleistocene, three monospecific genera are currently valid: Macrauchenia Owen, Reference Owen1838; Macraucheniopsis Paula-Couto, Reference Paula-Couto1945; and Xenorhinotherium Cartelle and Lessa, Reference Cartelle and Lessa1988 (Bond, Reference Bond1999; Schmidt and Ferrero, Reference Schmidt and Ferrero2014). The genus Xenorhinotherium, the focus of this study, occurred in Late Pleistocene–early Holocene. Its record is mainly in the Brazilian Intertropical Region (BIR), and it has also been recorded in Mato Grosso State and Venezuela, but these two occurrences of X. bahiense need to be revised (Cartelle and Lessa, Reference Cartelle and Lessa1988; Cartelle, Reference Cartelle1999; Salles et al., Reference Salles, Cartelle, Guedes, Boggiani, Janoo and Russo2006; Socorro, Reference Socorro2006). Moreover, we do not agree with the nomenclatural action by Guérin and Faure (Reference Guérin and Faure2004), which assigned X. bahiense Cartelle and Lessa, Reference Cartelle and Lessa1988 as a junior synonym of Macrauchenia patachonica Owen, Reference Owen1838. First, Guérin and Faure (Reference Guérin and Faure2004) did not compare their sample from caves in Piauí State with the type material of X. bahiense. Second, Guérin and Faure (Reference Guérin and Faure2004) did not contest any diagnostic features of X. bahiense as originally assigned by Cartelle and Lessa (Reference Cartelle and Lessa1988). Instead, Guérin and Faure (Reference Guérin and Faure2004) deemed the observed variation between X. bahiense and M. patachonica species-level variation without properly testing such an assumption. Indeed, other studies (e.g., Scherer et al., Reference Scherer, Pitana and Ribeiro2009) do not follow the taxonomic arrangement of Guérin and Faure (Reference Guérin and Faure2004), and further phylogenetic studies of Schmidt and Ferrero (Reference Schmidt and Ferrero2014) and Forasiepi et al. (Reference Forasiepi, MacPhee, Del Pino, Schmidt, Amson and Grohé2016) refuted the idea by revealing consistent morphological features that differ between the two monospecific genera.
Initially, the BIR paleogeographic region of X. bahiense occurrence was defined as characterized by dry forests, Cerrado and Caatinga ecosystems (sensu Cartelle, Reference Cartelle1999). However, with input of new information from palynological and carbon isotope data, the definition of BIR was refined as a mixed environment of Atlantic and dry tropical forest, with a latitudinal difference that influenced the vegetation structure (Werneck et al., Reference Werneck, Costa, Colli, Prado and Sites2011; Dantas et al., Reference Dantas, Dutra, Cherkinsky, Fortier, Kamino, Cozzuol, Ribeiro and Vieira2013).
Tooth eruption sequence is a topic of important concern for the study of ungulates, extant and extinct, due to its use in phylogenetic reconstructions and description of life history (Smith, Reference Smith2000). In fact, this topic brought to light a discussion of SANU’s relationship with the high-level extant clades, in which Agnolin and Chimento (Reference Agnolin and Chimento2011) claimed the close relationship of two SANU orders, Notoungulata and Astrapotheria, with Afrotheria. This hypothesis was based on their interpretation of three morphological characters: thoracolumbar vertebrae, late replacement of deciduous cheek teeth, and the presence of a fossa on astragalus. However, Billet and Martin (Reference Billet and Martin2011) and Kramarz and Bond (Reference Kramarz and Bond2014) examined material of three among the five SANU orders (Notoungulata, Astrapotheria, and Pyrotheria), and found no evidence to support an afrotherian-like delayed dental eruption in notoungulates, astrapotheres, and pyrotheres, contrary to the statement by Agnolin and Chimento (Reference Agnolin and Chimento2011). Moreover, proteomic studies using collagen proteins to perform phylogenetic analysis suggested that SANU is within Laurasiatheria and closely related to Perissodactyla (Welker et al., Reference Welker, Collins, Thomas, Wadsley, Brace, Cappellini, Turvey, Reguero, Gelfo, Kramarz, Burger, Thomas-Oates, Asford, Ashton, Rowsell, Porter, Kessler, Fischer, Baessmann, Kaspar, Olsen, Kiley, Elliott, Kelstrup, Mullin, Hofreiter, Willerslev, Hublin, Orlando, Barnes and MacPhee2015). Regarding the dental sequence of replacement for litopterns, the only data were provided by Bergqvist (Reference Bergqvist2010), but they are restricted to the succession of the third and fourth premolars, and from Paleocene litopterns from Brazil.
The pattern of dental replacement may indicate whether the species lived and died on a fast or a slow time scale (Smith, Reference Smith2000). However, the mammalian dentition also should provide other information by the classification of a tooth crown. This classification, at the hypsodonty level, could be determined by an index, expressing the ratio of tooth dimension (Janis, Reference Janis1988). Nevertheless, the index of hypsodonty brings information about crown height, and, consequently, relates to mode of feeding. Its information has to be aligned with other aspects, such as habitat type and other morphological features, to provide insight about species diet (Damuth and Janis, Reference Damuth and Janis2011). Most characterizations of crown height in Macraucheniidae refer to Macrauchenia patachonica Owen, Reference Owen1838, and, in a subjective way, are characterized as more hypsodont macraucheniid (Cifelli, Reference Cifelli1985) or are just cited as hypsodont cheek teeth (Paula-Couto, Reference Paula-Couto1979). Aside from these instances, there are no rigorous classifications for the tooth crowns of Pleistocene macracheniids (Paula-Couto, Reference Paula-Couto1979; Cifelli, Reference Cifelli1985). Consequently, the family needs a classification for phylogeny or morphological comparison, as is available for Protherotheriidae (Bond et al., Reference Bond, Perea, Ubilla and Tauber2001).
In the present study, we describe the eruption sequence of permanent upper dentition of the Pleistocene macraucheniid Xenorhinotherium bahiense using the juvenile paratype to evaluate if this species, a Late Pleistocene SANU, shows a delay on dental eruption. In addition, we determine the hypsodonty status of X. bahiense in order to clarify its ecological classification in the RIB biome, so providing information of this taxon to test hypotheses of relationship within the family.
Materials and methods
All the specimens analyzed came from Toca dos Ossos limestone cave, located at the municipally Ourolândia (UTM 24K 275224, 8790872), Bahia State, Brazil. Cartelle and Lessa (Reference Cartelle and Lessa1988) stated that the fossils were deposited at the cave during the Late Pleistocene to early Holocene.
Anatomical abbreviations
C=upper canine; d=deciduous; HI=hypsodonty index; I=upper incisive; P, p=upper and lower premolars, respectively; M, m=upper and lower molars, respectively.
Repository and institutional abbreviation
The material is housed in the MCL (Museu de Ciências Naturais da Pontifícia Universidade Católica de Minas Gerais, Belo Horizonte).
Description of eruption sequence
The documentation of the dental ontogeny of X. bahiense is based on the juvenile paratype (MCL 2643), which has an upper jaw with nine deciduous teeth and 17 permanent teeth. We also analyzed eight jaws, three uppers (MCL 2644/01, 3546, 3549) and five lowers (MCL 3559, 3560, 3577, 3658, 3769), of adult specimens, observing the wear stages of fully erupted permanent teeth in order to compare the sequence of replacement in the juvenile specimen.
Qualitative macroscopic analysis was performed in all teeth to describe their ontogeny, evaluating three features: eruption stage, development of tooth’s root, and degree of wear. For the eruption stage, we assigned four stages: “not erupted” (with germ in its dental crypt); “erupting” (below alveolar margin); “erupting above alveolar margin”; and “fully erupted.” For the development of the tooth root, we assigned three stages: “root fully opened” (with neck that may be delimited); “root start closing” (neck and roots are well delimited); and “root closed” (all roots fully formed and closed). For the degree of wear, we assigned five wear stages for each dentition that had been delimited by a combination of occlusal structure features, seen in upper (Fig. 1.1), and lower (Fig. 1.2) dentition. For upper teeth, the stages range from “no wear” (stage 1) to “extremely worn” (stage 5); when cusps are completely worn, they have straight mesial and distal margins, and mesiolabial fossette is vestigial or absent (Fig. 2). The three intermediate stages are: “little wear” (stage 2), when mesial and median structures are little worn but distal structures (metacone, metastyle) not are worn; “intermediate wear” (stage 3), when all cups and styles are worn and the mesial and distal cingulum are still present; “heavy worn” (stage 4), when the mesiolabial fossette is shallow, the mesial cingulum is absent, and the distal cingulum is vestigial or absent. For lower teeth, the stages range from “no wear” (stage 1) to “cuspids extremely worn out” (stage 5), when all structures are completely worn and forming a continuous flat surface (Fig. 2). The three intermediate stages are: “little wear” (stage 2), when the paralophid, protoconid, and metalophid (labial trigonid structures) are more worn than metaconid and all talonid structures; “intermediate wear” (stage 3), when trigonid structures show the same level of wear, the talonid structures are less worn than trigonid, and the metaconid and cristid oblique still are independent; “heavy wear” (stage 4), when the trigonid and talonid are deeply worn and join the metaconid and cristid obliquely.
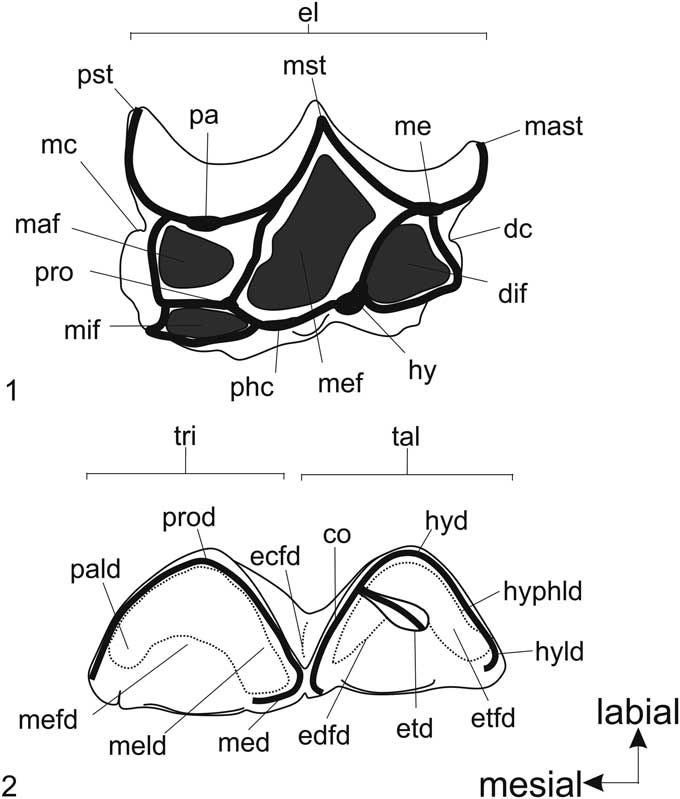
Figure 1 Structures of the cheek teeth of Xenorhinotherium bahiense. Abbreviations for upper cheek teeth (1): dc, distal cingulum; dif, distolingual fossette; el, ectoloph; hy, hypocone; maf, mesiolabial fossette; mast, metastyle; mc, mesial cingulum; me, metacone; mef, median fossette; mif, mesiolingual fossette; mst, mesostyle; pa, paracone; phc, protocone-hypocone crest; pro, protocone; pst, parastyle. Abbreviations for lower cheek teeth (2): co, cristid oblique; ecfd, ectoflexid; edfd, endoflexid; etd, entoconid; etfd, entoflexid; hyd, hypoconid; hyld, hypoconulid; hyphld, hypolophulid; med, metaconid; meld, metalophid; mefd, metaflexid; pald, paralophid; prod, protoconid; tal, talonid basin; tri, trigonid.
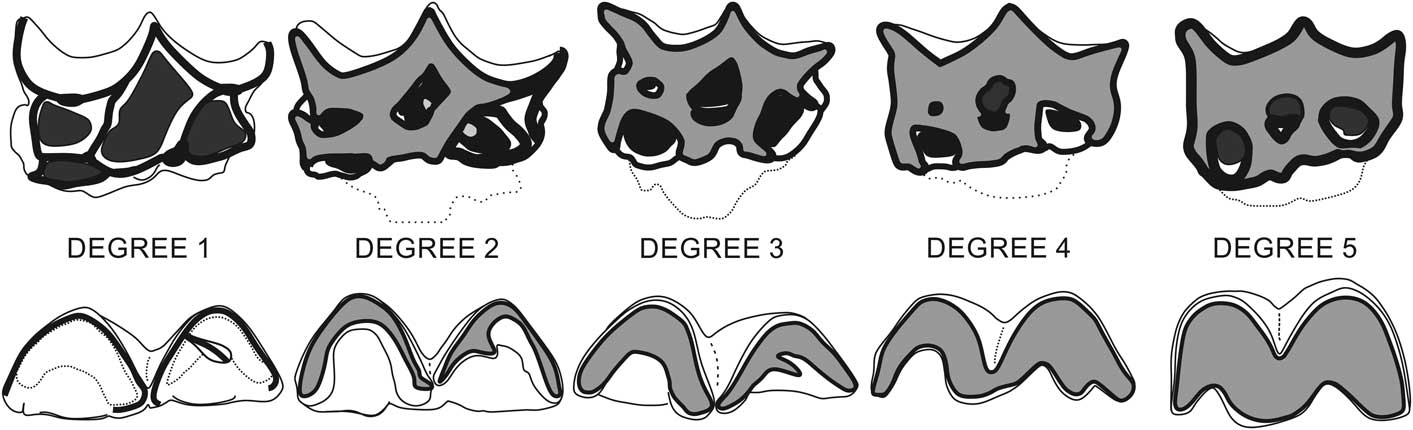
Figure 2 Wear degrees of Xenorhinotherium bahiense in upper (above) and lower (below) teeth. With wear progression, the enamel (highlighted in white) is worn and the dentine (highlighted in gray) is progressively exposed, whereas upper teeth fossettes (represented in black) become apparently smaller due to their conical shape until they disappear (see Figure 1 for structures identification).
Hypsodonty index
For the classification of X. bahiense, we used the hypsodonty index (HI; Janis Reference Janis1988), which is the ratio between height and width of tooth crown. This index is calculated only for third lower molars that do not exhibit excessive dental wear. Therefore, the HI in X. bahiense is based on five specimens (MCL 3558, 3772, 3776, 3795, 3839), which are classified in stages 1 and 2. All the measurements are in millimeters (mm).
Results
Eruption sequence
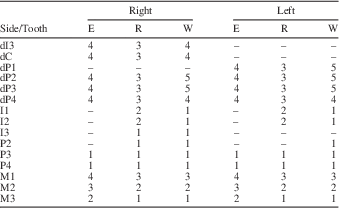
The dental remains of MCL 2643 (Xenorhinotherium bahiense paratype) are composed of two fragmented upper jaws with cheek teeth and 17 isolated teeth, adding up to 26 teeth belonging to this juvenile individual (Fig. 3). The right dentition contains dP3–M3 in a fragmentary upper jaw, and dI3–dC, dP2, I1–I3, and P2 isolated; also, the alveolar crypt of P3 and P4 is preserved. The left dentition contains dP4–M3 implanted in a fragmentary upper jaw, and dP1–dP3, I1–I2, and P2–P4 isolated, and the alveolar crypt of P4. The two sides of the paratype dentition exhibit the same dental features (Table 1). The two deciduous teeth exhibit distinct degrees of wear, with dP3 more worn than dP4. This makes it possible to determine that dP3 erupted before dP4. For the incisors, I1 and I2 exhibit roots in the same stage of development as the root of M2, which indicates that the I1 and I2 erupted before M3. All other premolars exhibited the same parameters examined, suggesting that all premolars erupted in a short period. Although there is a lack of information on permanent premolar eruption sequence, we obtained some information on the permanent eruption sequence through an analysis of deciduous premolars. The occlusal surfaces of the dP1–dP3 are completely worn, and even the dP4 is considerably worn, with occlusal structures still present and outlined by enamel (fossettes mesiolingual, median, and distolingual). This indicates that dP1–dP3 lost their function, whereas dP4 was still functional, therefore P1–P3 should have erupted before P4. Due to the absence of both C, their positions in the replacement sequence cannot be assessed. Therefore, we assumed that the sequence of replacement of permanent dentition in this juvenile specimen was M1>M2>I1>I2>M3>I3>P1>P2>P3>P4.

Figure 3 Upper dentition of juvenile specimen of Xenorhinotherium bahiense, with deciduous and permanent teeth (MCL 2643). (1–13), right teeth; third deciduous incisor, (1) labial and (2) lingual views; deciduous canine, (3) labial and (4), lingual views; second deciduous premolar, (5) labial and (6) lingual views; first permanent incisor, (7) lingual view; second permanent incisive, (8) lingual view; fragmentary right jaw with dP3-M3 implanted, (9) occlusal view; third permanent incisor, (10) lingual view; second permanent premolar, (11) occlusal view; third permanent premolar, (12) occlusal view; fourth permanent premolar, (13) occlusal view; (14–25), left teeth; first deciduous premolar, (14) labial and (15) lingual views; second deciduous premolar, (16) labial and (17) lingual views; third deciduous premolar, (18) labial and (19) lingual views; first permanent incisor, (20) lingual view; second permanent incisor, (21) lingual view; second permanent premolar, (22) occlusal view; third permanent premolar, (23) occlusal view; fourth permanent premolar, (24) occlusal view; fragmentary left jaw with dP4-M3, (25) occlusal view. Scale bar=2 cm.
Table 1 Development of each tooth from the specimen MCL 2643; E=eruption stage; R=root development; W=degree of wear; –=missing data.
The dentition of MCL 2644 (right and left) in the skull of the holotype has the right I1–M3 and left P1–M3 (Fig. 4.1). The only incongruence within the eruption sequence is that the P4 exhibit the same wear stage of M1. The sequence I1–C exhibits the same level of wear, with the paracone worn but still keeping its shape. Other upper dentitions of adult individuals, MCL 3546 and MCL 3549, were also examined (Fig. 4.2, 4.3). As observed for MCL 2644, the P4 were more worn than M3 in all adult specimens. MCL 3546, a specimen with teeth less worn than in the other specimens, has P4 less worn than M1. This indicates that the wear stages are influenced by other factors besides the sequence eruption of the teeth.

Figure 4 Dentition (occlusal view) of adult specimens of Xenorhinotherium bahiense. (1) MCL 2644; (2) MCL 3549; (3) MCL 3546; (4) MCL 3577. Scale bar=2 cm.
MCL 3577 is a fragmented lower jaw with the dental sequence p2–m3 (Fig. 4.4). Even the p2 is less molarized than the other premolars in this specimen, and it is evident that p2 is less worn than the other premolars. That interpretation is supported by the wear on the protoconid, metaconid, and cristid oblique. Also, p4 is less worn than m1, which is supported by the wear on the metaconid. However, as in the upper dentition of analyzed adult specimens, p4 is more worn than m3, which is supported by the metaconid, and the talonid, which shows no wear on m3. Other specimens that have lower teeth studied are MCL 3559, MCL 3560, MCL 3658, and MCL 3769. All of them exhibit the same pattern as described for MCL 3577, but with less information because these specimens preserve fewer teeth.
Hypsodonty index
The sample comprises two m3 with no wear (stage 1) and three m3 with little wear (stage 2) (Table 2). The mean of m3 with no wear is 2.24; the mean including the other three m3 (stage 2) is 2.04. Both results place X. bahiense close to the interval of 1.50–3.00, thus characterizing the species as having mesodont crowns.
Table 2 Measurements of specimens for calculation of hypsodonty index (HI).

Discussion
Eruption sequence
The results indicate that X. bahiense has a sequence of replacement most similar to a rapid-growth mammal, with incisors and premolars erupting after the molars (Smith, Reference Smith2000). The extant terrestrial ungulates with most similar sequence of replacement are the artiodactyls, specifically species of families Cervidae and Giraffidae, such as Cervus elaphus Linnaeus, Reference Linnaeus1758 and Okapia johnstoni (Sclater, Reference Sclater1901), respectively (Smith, Reference Smith2000). On the other hand, the currently accepted phylogenetic hypotheses for placental mammals (Buckley, Reference Buckley2015; Welker et al., Reference Welker, Collins, Thomas, Wadsley, Brace, Cappellini, Turvey, Reguero, Gelfo, Kramarz, Burger, Thomas-Oates, Asford, Ashton, Rowsell, Porter, Kessler, Fischer, Baessmann, Kaspar, Olsen, Kiley, Elliott, Kelstrup, Mullin, Hofreiter, Willerslev, Hublin, Orlando, Barnes and MacPhee2015) place SANU as more closely related to perissodactyls. This divergence between phylogenetic signal and dental eruption sequence suggests that an ecological pressure on this trait was possibly important during ungulate evolution, with some cases of adaptive convergence in dental eruption sequences (Smith, Reference Smith2000).
Our results have only a single incongruence in wear sequence between juvenile and adult specimens. The wear stage of P4 differs from that expected for adult specimens. Therefore, the wear stage of P4 in adult X. bahiense (MCL 2644, MCL 3546) is greater than that of M3. However, the presence of the dental crypt in P4 and the stage of development of its root establish its placement in the eruption sequence. The incongruence observed in adult wear stage of P4 is probably due to the masticatory process, as suggested by Lessa (Reference Lessa1992), in which X. bahiense has M3 less subject to wear than other cheek teeth. In addition, the angle of occlusion between the upper and lower jaws could cause this difference in wear rate among teeth that differ in positions (Spinage, Reference Spinage1971).
The only data about the permanent eruption sequence for Litopterna is provided by Bergqvist (Reference Bergqvist2010) using species of the families Protolipternidae and Proterotheriidae: Protolipterna ellipsodontoide Cifelli, Reference Cifelli1983 and Paranisolambda prodromus (Paula-Couto, Reference Paula-Couto1952), respectively. Bergqvist’s data provides information about the replacement sequence between the two posterior premolars (P3 and P4) and the molar sequence. Although the data of P. ellipsodontoide and P. prodromus are incomplete, a clear distinction is observed between them and the sequence for X. bahiense: the P3 and P4 erupted before or almost at the same time as M3 (Bergqvist, Reference Bergqvist2010), whereas in X. bahiense P3 and P4 erupted after M3. Furthermore, in P. ellipsodontoide P4 erupted before P3. This is contrary to that observed for X. bahiense in our study and Phenacodontidae, a family of North American “archaic ungulates” closely related to Litopterna (West, Reference West1981).
The “delayed dental eruption” has two concepts clarified by Billet and Martin (Reference Billet and Martin2011). Our focus here is on the concept of delayed eruption of permanent dentition relative to skull growth, which is regarded as a synapomorphy of Afrotheria (Asher and Lehmann, Reference Asher and Lehmann2008). This delay of eruption occurs when one species spends well over half of its lifespan without a completely erupted permanent dentition (e.g., elephants, sea-crows, and hyraxes) (Asher and Lehmann, Reference Asher and Lehmann2008). Although we do not consider cranial measurements in our sample, they explain, the absence of delayed dental eruption in the juvenile individual (MCL 2643). This is evident because this juvenile individual (MCL 2643) has already erupted most of its permanent dentition. Moreover, the juvenile does not show co-ossified epiphyses on the limb bones as completely ossified, and the fusion between radius and ulna had yet not begun (Cartelle and Lessa, Reference Cartelle and Lessa1988; Lessa, Reference Lessa1992). In summary, our results reinforce the observation that South American native ungulates (SANU) do not show delayed dental eruption, as previously suggested by Billet and Martin (Reference Billet and Martin2011) and Kramarz and Bond (Reference Kramarz and Bond2014) for other orders of SANU. This rejects the proposed diagnostic synapomorphy of SANU and Afrotheria by Agnolin and Chimento (Reference Agnolin and Chimento2011).
The closest related litopterns in which the HI has been applied are species of the family Proterotheriidae. The sampled species show a pattern of increase of the HI during the diversification of the family in the Cenozoic (Bond et al., Reference Bond, Perea, Ubilla and Tauber2001). The HI in Proterotheriidae changes from brachyodont teeth in late Oligocene–middle Miocene species to mesodont teeth in late Miocene species, and reaches the highest value (2.00–2.50) in Pleistocene species with Neolicaphrium recens Frenguelli, Reference Frenguelli1921 (Bond et al., Reference Bond, Perea, Ubilla and Tauber2001). The best-known families of Litopterna, Macraucheniidae, and Protherotheriidae are represented by species with mesodont teeth in the Pleistocene (Bond, Reference Bond1999).
Damuth and Janis (Reference Damuth and Janis2011) reported that HI should only be interpreted by taking into account the habitat type, as the Brazilian Intertropical Region is considered a region in which Atlantic and dry forest vegetation could occur. Due to the variation in habitat type in RIB, we suggest that the environment occupied by X. bahiense includes woodland, an open forest where canopy is still mostly continuous but ground cover may include grass, forest, and a closed forest, with few or no clearings (Werneck et al., Reference Werneck, Costa, Colli, Prado and Sites2011; Dantas et al., Reference Dantas, Dutra, Cherkinsky, Fortier, Kamino, Cozzuol, Ribeiro and Vieira2013). Using this relation between HI and habitat type, X. bahiense has been characterized as a browser, with diet based on C3 plants, in both habitat types. This interpretation is different from the diet hypothesized for the other Late Pleistocene macraucheniid. Macrauchenia patachonica based on isotope analysis as a mixed feeder, with a diet based on plants C3 and C4 (Domingo et al., Reference Domingo, Prado and Alberdi2012). Additionally, Janis (Reference Janis1995) proposed that the shape of premaxilla could be useful as an indicator of diet in terrestrial herbivores. This observation corroborates our proposal for differences in diet between X. bahiense and M. patachonica because both species show different premaxilla shapes—sharpened and straight, respectively (Cartelle and Lessa, Reference Cartelle and Lessa1988; Schmidt and Ferrero, Reference Schmidt and Ferrero2014).
Future studies should focus on other litopterns, mainly Adianthidae and Proterotheriidae, families that are closely related with Macraucheniidae (Cifelli, Reference Cifelli1985), to evaluate whether there is a common pattern of replacement sequence in monophyletic groups or whether there are multiple sequences during macraucheniid evolution, as in Artiodactyla and Perissodactyla (Smith, Reference Smith2000). Furthermore, it is necessary to add more data about HI for Macraucheniidae in order to evaluate whether the increase in hypsodonty occurs with the diversification of the lineage, as in North American horses and probably in Proterotheriidae (Bond et al., Reference Bond, Perea, Ubilla and Tauber2001; Damuth and Janis, Reference Damuth and Janis2011). Additionally, data from other approaches (e.g., microwear and isotope analysis) are needed to make inferences concerning the paleoecology of Macraucheniidae.
Acknowledgments
We thank Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for the financial grant and Universidade Federal de Viçosa for the physical support to develop this work (grants to G. Lessa). This paper includes part of the Master’s thesis of L. Lobo and was supported by CAPES. A.M. Ribeiro (FZB/RS) was part of the thesis evaluating committee and provided important suggestions that improved the manuscript. L. Lobo also thanks CAPES for his current scholarship (PhD candidate). We thank G. Muricy (Museu Nacional/Universidade Federal do Rio de Janeiro) for his critical revision of earlier versions of the manuscript. We also thank M. Bond, a second anonymous reviewer, and the editors for suggestions and comments.








