Introduction
A relatively small crinoid fauna is described herein from the Wooster Shale Member of the Cuyahoga Formation from Wayne and Ashland counties, Ohio, USA (Mississippian, Tournaisian). Although distinct (five new species), the Wooster Shale fauna is similar to the fauna of the stratigraphically lower Meadville Shale Member of the Cuyahoga Formation. The Wooster Shale Member fauna also has a much lower biodiversity that the Meadville Shale Member (Tables 1 and 2). Both faunas contain actinocrinitids, platycrinitids, Taxocrinus, Cyathocrinites, and decadocrinids. The only shared species between these faunas is Cyathocrinites simplex Kammer and Roeser, Reference Kammer and Roeser2012. Five new species are described herein from the Wooster Shale Member: Cactocrinus woosterensis n. sp., Cusacrinus brushi n. sp., Agaricocrinus murphyi n. sp., Decadocrinus laevis n. sp., and Decadocrinus inordinatus n. sp. In addition, Megistocrinus? sp. is also described from the Wooster Shale Member. Unlike the Meadville Shale Member crinoids, many of the Wooster Shale Member crinoids are completely or partially preserved with siderite. Crinoids associated with siderite occur either in siderite nodules or within siderite beds. The paleoenvironmental setting and faunal content of these two Cuyahoga Formation crinoid faunas are in many ways more similar to Viséan faunas in siliciclastic settings than to other Tournaisian faunas. Despite being nearly contemporaneous, clade-specific occurrences of new species is consistent with species longevity distinctions discussed by Kammer et al. (Reference Kammer, Baumiller and Ausich1997, Reference Kammer, Baumiller and Ausich1998).
Table 1. Members of the Cuyahoga Formation in northeastern Ohio with approximate thicknesses; data from Szmuc, Reference Szmuc1970; Coogan et al., Reference Coogan, Heimlich, Malcuit, Bork and Lewis1981; Matchen and Kammer, Reference Matchen and Kammer2006 (modified from Kammer and Roeser, Reference Kammer and Roeser2012).

Table 2. Comparison of the crinoid faunas in the Wooster Shale and Meadville Shale members of the Cuyahoga Formation. Meadville Shale Member data from Ausich and Roeser (Reference Ausich and Roeser2012), Kammer and Roeser (Reference Kammer and Roeser2012), and Webster (Reference Webster2014).

Geologic setting
Stratigraphy
The Wooster Shale Member is a member of the Cuyahoga Formation in northeastern and central Ohio (Table 1). Its age has been debated. Szmuc (Reference Szmuc1957), in his initial description of the member, and Rodriquez (Reference Rodriquez1961) suggested that the Wooster Shale Member was later Kinderhookian to early Osagean based on its brachiopod fauna. Szmuc (Reference Szmuc1970) further described the members of the Cuyahoga Formation, noting that the Wooster Shale Member was distinguished by its homogenous dark shale with abundant fossiliferous concretions dominated by large syringothyrid brachiopods and platyceratid snails. Using miospores, Clayton et al. (Reference Clayton, Manger and Owens1998) confirmed that the Wooster Shale Member was middle Tournaisian and likely late Kinderhookian. Matchen and Kammer (Reference Matchen and Kammer2006) suggested that the Wooster Shale Member was deposited during a transition between the Kinderhookian and Osagean, represented by a hiatus of two conodont zones in the Mississippi River Valley (see also Kammer and Matchen, Reference Kammer and Matchen2008). In any case, we are confident that the Wooster Shale Member is middle Tournaisian in global stratigraphic terminology.
Depositional environment
The Wooster Shale Member, with its diverse fauna of brachiopods, crinoids, and mollusks, is clearly marine in origin. Clayton et al. (Reference Clayton, Manger and Owens1998) noted that the abundance of terrestrial kerogens in the member at its type locality in Wooster, along with miospore tetrads and megaspores, indicates that it was deposited in a nearshore environment. Specifically, Clayton et al. (Reference Clayton, Manger and Owens1998, p. 190) suggested that the occurrences of acanthomorph acritarchs, tasmanitids, and Botryococcus in the Wooster Shale Member “are consistent with deposition in an interdistributary setting associated with a true deltaic environment.”
Materials and methods
Specimen collection
The Wooster Shale Member of the Cuyahoga Formation is exposed primarily along small streams in Wayne and Ashland counties, Ohio. These outcrops confirm that Wooster Shale Member crinoids are preserved encased in gray shale or associated with siderite concretions, matching the specimens in museum collections. However, bedding-plane exposures of the shale are very limited in stream banks, so few identifiable remains have been recovered from these outcrops.
The crinoids described here are primarily from historical collections from the College of Wooster; the Orton Geological Museum, The Ohio State University (James L. Murphy collection); the Cleveland Museum of Natural History (Gary Meszaros collection); and Ashland College (Nigel Brush collection). None of the primary localities of these collections remains accessible for collection. Most specimens are from the abandoned Medal Brick and Tile Quarry in Wooster. Other specimens described here are from an abandoned shale pit in Ruggles Township (Ashland County), a grassed-over Interstate 71 outcrop in Wayne County, and an outcrop along Shade Creek in Wayne County (Fig. 1). There can be little expectation of collecting many well-preserved new specimens unless new shale quarries are opened in the future.

Figure 1. Locality map in northeastern Ohio for crinoid occurrences in the Wooster Shale Member; approximate positions indicated by stars (as discussed in text, these localities are no longer accessible for collection): 1—abandoned Medal Brick and Tile Quarry, Wooster, Wayne County, Ohio; 2—Shade Creek in Wayne County, Ohio; 3—grassed-over roadcut along Interstate 71, south of County Road 126, sec. 10, Congress Township, Wayne County; 4—abandoned shale pit ~1.5 miles south of New London, east side of Highway 60, Ruggles Township, Ashland County, Ohio.
Repositories and institutional abbreviations
New specimens reported in this study are deposited at the Cleveland Museum of Natural History, Cleveland, Ohio (CMNH) and in the Orton Geology Museum, The Ohio State University, Columbus, Ohio (OSU).
Systematic paleontology
Classification and terminology
Ordinal and superordinal classification of crinoids follows Cole (Reference Cole2017, Reference Cole2018), Wright (Reference Wright2017a, Reference Wrightb), and Wright et al. (Reference Wright, Ausich, Cole, Rhenberg and Peter2017). Family-level classification follows Moore and Teichert (Reference Moore and Teichert1978). Morphologic terminology is from Webster (Reference Webster1974), Ubaghs (Reference Ubaghs, Moore and Teichert1978a), Webster and Maples (Reference Webster, Maples, Ausich and Webster2008), Kammer et al. (Reference Kammer, Sumrall, Zamora, Ausich and Deline2013), Ausich et al. (Reference Ausich, Wright, Cole and Sevastopulo2020), and Ausich and Donovan (Reference Ausich, Donovan and Ausichin press). Interray plating is indicated by the number of plates in each range from the proximal-most plate to the last range before the tegmen (e.g., 1-2-2-1). In the posterior interray, the primanal is designated by “P,” and in regular interrays the proximal-most plate is designated by “1.” A, B, C, D, and E represent echinoderm rays following the Carpenter Ray system (see Ubaghs, Reference Ubaghs, Moore and Teichert1978a, p. T63). Heteromorphic column patterns are indicated using the Webster (Reference Webster1974) system.
In specimen measurements, abbreviations are as follows: ACH, aboral cup height; ACW, aboral cup width; CaH, calyx height; CaW, calyx width; ColH, column height; CrH, crown height. All measurements are in mm; * after a measurement indicates the specimen is crushed or the feature is incomplete.
Sources for the list of species included in each genus differ among genera. For Cactocrinus and Cusacrinus, the included species are from a comprehensive review of the Actinocrinitidae (Rhenberg et al., Reference Rhenberg, Ausich and Kammer2015). Species lists in the other genera are taken uncritically from Webster (Reference Webster2014).
Class Crinoidea Miller, Reference Miller1821
Subclass Camerata Wachsmuth and Springer, Reference Wachsmuth1885
Infraclass Eucamerata Cole, Reference Cole2017
Order Monobathrida Moore and Laudon, Reference Moore and Laudon1943
Suborder Compsocrinina Ubaghs, Reference Ubaghs, Moore and Teichert1978
Family Periechocrinidae Bronn, Reference Bronn1849
Genus Megistocrinus Morris, Reference Morris1843
Type species
Megistocrinus evansii (Owen and Shumard, Reference Owen and Shumard1850).
Included species
Megistocrinus abnormis (Lyon, Reference Lyon1857); M. broadheadi Branson and Wilson, Reference Branson and Wilson1922; M. circulus Rowley, Reference Rowley1904b; M. clarkei Thomas, Reference Thomas1924; M. concavus Wachsmuth, Reference Wachsmuth1885; M. corniger Rowley, Reference Rowley1901b; M. depressus (Hall, Reference Hall1862); M. devonicus Charlesworth, Reference Charlesworth1914; M. evansii (Owen and Shumard, Reference Owen and Shumard1850); M. evansii crassus White, Reference White1862; M. expansus Miller and Gurley, Reference Miller and Gurley1894; M. expansus inflatus Rowley, Reference Rowley1901b; M. expansus magniventrus Rowley, Reference Rowley1903a; M. farnsworthi White, Reference White1876; M. fitzpatricki Thomas, Reference Thomas1924; M. hemisphericus Miler and Gurley, Reference Miller and Gurley1895; M. indianensis Miller and Gurley, Reference Miller and Gurley1896a; M. knappi (Lyon, Reference Lyon1862); M. latus (Hall, Reference Hall1858); M. merrilli Thomas, Reference Thomas1924; M. mineolaensis Branson and Wilson, Reference Branson and Wilson1922; M. missouriensis Branson and Wilson, Reference Branson and Wilson1922; M. multidecoratus (Barris, Reference Barris1886); M. nobilis Wachsmuth and Springer in Miller, Reference Miller1889; M. nodosus Barris, Reference Barris1880; M. novus (Wood, Reference Wood1904); M. ontario (Hall, Reference Hall1862); M. oppelti Rowley, Reference Rowley1903c; M. ornatus Miller and Gurley, Reference Miller and Gurley1895; M. pernodosus Thomas, Reference Thomas1924; M. reeftonensis Prokop, Reference Prokop1970; M. regularis Wood, Reference Wood1904; M. robustus Thomas, Reference Thomas1924; M. rugosus Lyon and Casseday, Reference Lyon and Casseday1859; M. rugosus spinuliferus Rowley, Reference Rowley1903a; M. sphaeralis Wood, Reference Wood1904; M. spinosulus Lyon, Reference Lyon1862; M. tuberatus Wood, Reference Wood1904; M. unicornis Rowley, Reference Rowley1901b; and M. whitehalli Laudon, Reference Laudon1973.
Megistocrinus? sp.
Figure 2.1
Occurrence
Mississippian (Tournaisian) Wooster Shale Member, Cuyahoga Formation at the abandoned Medal Brick and Tile Quarry, Wooster, Wayne County, Ohio.

Figure 2. Wooster Shale Member camerate crinoids. (1) CMNH 18011, partial crown of Megistocrinus? sp. completely replaced by siderite. (2–4, 6) Cactocrinus woosterensis n. sp.; (2) OSU 53550, paratype, specimen in a siderite concretion (compare to Fig. 6.2); (3) CMNH 5212, holotype, somewhat collapsed crown with the proximal portion of the column attached; note small plates in calyx with variable sculpturing; (4) CMNH 18012, paratype, complete set of arms with very poorly preserved calyx; (6) CMNH 5212, holotype, enlargement of the arms at mid-height illustrating spines on pinnulars. (5) Cusacrinus brushi n. sp., CMNH 18014, poorly preserved partial crown of that is at least partially replaced with siderite and with an attached Platyceras gastropod. Scale bar represents 5.0 mm in (1, 2, 5, 6) and 10.0 mm in (3, 4). Specimens in (2) and (6) coated with ammonium chloride sublimate for photography.
Material
CMNH 18011.
Measurements
CMNH 18011: CaH, 24.0*; CaW, 26.0*; AH, 4.0*.
Remarks
Approximately one-eighth of an articulated, uncompacted calyx is preserved in siderite surrounded by gray shale (CMNH 18011). Some secundibrachials through quartibrachials are fixed into the calyx (Fig. 2.1). A median ray ridge is present from at least the fixed tertibrachials through the remaining fixed brachials. The distal portion of one half-ray is well preserved, which has four arms. The outer half of the half-ray is unbranched, whereas the inner portion of the half-ray branches twice. The distal-most fixed brachials are uniserial, but the free arms are biserial.
This specimen is too incomplete to assign to a genus and species with confidence, but the morphology that is preserved is consistent with that of Megistocrinus. Megistocrinus is primarily a Devonian camerate crinoid; however, two species are present in Tournaisian strata of the United States. Megistocrinus evansii, which is the type species of this genus, is known from the lower Burlington Formation of Iowa and the Lake Valley Formation of New Mexico, and M. nobilis is known from the Maynes Creek Formation of Iowa. Both of these species have much larger and more robust calyx plates and, as known so far, do not have fixed quintibrachials. However, this specimen is too incomplete to be the basis of a new species.
Family Actinocrinitidae Austin and Austin, Reference Austin and Austin1842
Remarks
The Actinocrinitidae is a diverse, cosmopolitan family that was a dominant element of many Mississippian paleocommunities. The Wooster Shale Member of the Cuyahoga Formation contains two species belonging to the Actinocrinitidae: Cactocrinus woosterensis n. sp. and Cusacrinus brushi n. sp. These two species have sharply contrasting morphologies that are distinct from Cusacrinus dafne (Hall, Reference Hall1863), which occurs in the Meadville Shale Member of the Cuyahoga Formation (Ausich and Roeser, Reference Ausich and Roeser2012). Interestingly, all three of these species (as well as some other species in these genera) have peculiar arms (i.e., the pinnules have spines projecting from pinnular plates). As discussed below, both of the species conform well to the diagnosis of their respective genera in the recent revision of the Actinocrinitidae (Rhenberg et al., Reference Rhenberg, Ausich and Kammer2015), but the diagnoses of Rhenberg et al. (Reference Rhenberg, Ausich and Kammer2015) must be modified slightly to accommodate the inclusion of these two species.
Subfamily Cactocrininae Ubaghs, Reference Ubaghs, Moore and Teichert1978
Genus Cactocrinus Bowsher, Reference Bowsher1955
Type species
Actinocrinus proboscidialis Hall, Reference Hall1858, by original designation.
Included species
Cactocrinus arrosus (Miller, Reference Miller1892a); C. baccatus Wood, Reference Wood1914; C. bischoffi (Miller and Gurley, Reference Miller and Gurley1896b); C. clarus (Hall, Reference Hall1861a); C. extensus Wachsmuth and Springer, Reference Wachsmuth and Springer1897; C. fossatus (Miller, Reference Miller1892a); C. glans (Hall, Reference Hall1859); C. hurdianus (McChesney, Reference McChesney1860); C. imperator (Laudon, Reference Laudon1933); C. lucina (Hall, Reference Hall1861b); C. magnidactylus Laudon and Severson, Reference Laudon and Severson1953; C. multibrachiatus (Hall, Reference Hall1858); C. obesus (Keyes, Reference Keyes1894); C. opusculus (Hall, Reference Hall1859); C. platybrachiatus Wood, Reference Wood1914; C. proboscidialis (Hall, Reference Hall1858); C. sexarmatus (Hall, Reference Hall1859); C. springeri (Rowley, Reference Rowley1900); C. thalia (Hall, Reference Hall1861b); and C. woosterensis n. sp.
Diagnosis
Basal circlet low; one or two fixed secundibrachials, secundibrachials or tertibrachials highest brachitaxis in vertical wall of calyx; two or three ranges of interradial plates in regular interrays, three ranges of interradial plates in posterior interray, interrays not in contact with tegmen, plating in proximal interrays 1–2; fixed intrabrachials between half-rays; arms not grouped, arm lobes absent; free arms atomous; tegmen height similar to calyx height, comprised of many plates, anal tube central (modified from Rhenberg et al., Reference Rhenberg, Ausich and Kammer2015).
Occurrence
Mississippian (Tournaisian) of North America.
Remarks
Placement of C. woosterensis n. sp. in Cactocrinus is based on a low basal circlet, low radial circlet, number of ranges of plates in regular interrays, interrays not in contact with the tegmen, 1-2 plating in proximal regular interrays, arms not grouped, arm lobes absent, and fixed intrabrachials between half-rays (see Rhenberg et al., Reference Rhenberg, Ausich and Kammer2015). Inclusion of C. woosterensis n. sp. in Cactocrinus expands the diagnosis of this genus because C. woosterensis n. sp. has two fixed secundibrachials rather than only one.
Cactocrinus woosterensis new species
Figures 2.2–2.4, 2.6, 6.2–6.4
Types
Holotype: CMNH 5212; paratypes: CMNH 18012, CMNH 18013, OSU 53550.
Diagnosis
Radial plates wider than high: irregular radiating ridged sculpturing, sculpturing along rays inconsistent; first primibrachial larger than second primibrachial, two fixed secundibrachials, tertibrachials highest brachitaxis in vertical wall of calyx; interradial and intrabrachial plates with one large, central node, with a smaller node above (calyx shape, and characters of the tegmen unknown).
Occurrence
Mississippian (Tournaisian) Wooster Shale Member, Cuyahoga Formation at the abandoned Medal Brick and Tile Quarry, Wooster, Wayne County, Ohio; Shade Creek in Wayne County; and a grassed-over roadcut along I-71 south of County Road 126, SE1/4, NW1/4, sec. 10, Congress Township, Wayne County, Ohio.
Description
Crown large. Calyx large, cone shaped without a basal concavity (Fig. 2.3). Calyx plate sculpturing prominent but highly variable. Basal circlet ~10% of calyx height; basal plates presumably three, visible in lateral view; with narrow, undulating ridge around base of basal circlet, arcuate outer rim of facet with radial plate above, and a vertical ridge centered below the center of the facet for the radial plate and extending down to the rim at the base of the basal plate. Radial circlet ~20% of calyx height, presumably in lateral contact in all interrays except the CD interray; radial plates small, five, hexagonal, as wide as high. Radial plate sculpturing irregular and varied; vertical ridge from basal plate connects to a ridge on the radial plate that extends to the middle of the radial plate where it joins a dominant horizontal ridge, from this horizontal ridge various irregular ridges or nodes may be present with the pattern different on different radial plates.
Regular interrays not in contact with tegmen (Fig. 2.3), interradial plates small, hexagonal. Fixed interradial plates with a large central node covering most of plate and a smaller central node on top of the larger node. First interradial plate wider than high with prominent horizontal ridge. Regular interray plating 1-2-3-?. CD interray unknown.
Fixed brachials at least through the first quartibrachial; with various, irregular sculpturing; typically, prominent three, four, or five radiating ridges, a single horizontal ridge or a single node. Fixed brachials ~70% of calyx height; fixed intrabrachials between fixed secundibrachials, with a central spine.
Tegmen unknown.
Free arms 40, high, atomous (Fig. 2.3, 2.4), chisel biserial after the third quartibrachial, as wide as high and deeper than wide (Fig. 2.6). Pinnules long; pinnulars with spine projecting obliquely upward and more or less perpendicular to the pinnule attitude if arms closed (Fig. 2.6).
Column circular, heteromorphic, N212 pattern (Fig. 2.3). Nodal with prominent, wide, thin ridge around latus; priminternodal with a narrower, thin ridge around latus; secundinternodal with a flat latus.
Etymology
The species name recognizes the College of Wooster in Wooster, Ohio, for its decades of support of paleontological research. The holotype of Cactocrinus woosterensis n. sp. and many other specimens in this fauna were collected by professors and students from the Department of Geology at The College of Wooster.
Additional specimen
CMNH 18018.
Measurements
CMNH 5212: CrH, 110.0*; CaH, 27.0*, CaW, 54.0*; AH, 88.0*; ColH, 46.0*. CMNH 18012: CrH, 112.0*; CaW, 35.0*; AH, 93.0. OSU 53550: CaH, 19.1; CaW, 24.0*.
Remarks
The holotype of Cactocrinus woosterensis n. sp. is a nearly complete crown with ~48 mm of attached column. Unfortunately, the calyx is compacted with most of the calyx plates separated but basically in their proper relative positions. This means that the calyx shape and dimensions can only be approximated.
Twenty species are now recognized in Cactocrinus (see Rhenberg et al., Reference Rhenberg, Ausich and Kammer2015). These species can be subdivided into two major groups: 1, those species with ray plate sculpturing dominated by some arrangement of radiating ridges (single ridges connecting with like ridges of adjoining plates); and 2, those species with other types of plate sculpturing, such as smooth sculpturing or plates dominated by a single spine. Thirteen species have radiating ridges as the type of ray plate sculpturing, including C. woosterensis n. sp. (see Supplemental Table 1). Some of these species have a central node (sizes variable), but all have a more or less uniform character of radiating ridges along a ray.
Cactocrinus woosterensis n. sp. and Cactocrinus imperator (Laudon, Reference Laudon1933) are unique among Cactocrinus species because tertibrachials are the highest fixed brachials in the vertical wall of the calyx. These two species are distinguished because Cactocrinus woosterensis n. sp. has inconsistent plate sculpturing along ray plates, two fixed secundibrachials, and a distinctive double node sculpturing on fixed interradial plates. In contrast, C. imperator has similar plate sculpturing along ray plates, one fixed secundibrachial, and radiating ridges as plate sculpturing on fixed interradial plates.
One paratype of C. woosterensis n. sp. (CMNH 18012) (Fig. 2.4) is also oddly preserved with a complete set of arms and a compressed, poorly preserved calyx. OSU 53550, also a paratype, is a partial, uncompressed calyx preserved in a siderite nodule. In addition, partial specimens presumably assignable to Cactocrinus woosterensis n. sp. were observed (but not collected) at various stream exposures in Wayne and Ashland counties.
Two actinocrinitid camerates are present in the Wooster Shale Member of the Cuyahoga Formation. This contrasts with the Meadville Shale Member of the Cuyahoga Formation, which has only one actinocrinitid species, Cusacrinus daphne (Hall, Reference Hall1863) (Ausich and Roeser, Reference Ausich and Roeser2012). The primary, striking distinction between Cactocrinus and Cusacrinus is the size and number of calyx plates. The Cactocrinus calyx is constructed of numerous, small plates, whereas, Cusacrinus has relatively few, large calyx plates (Ausich and Roeser, Reference Ausich and Roeser2012).
Genus Cusacrinus Bowsher, Reference Bowsher1955
Type species
Actinocrinites proboscidialis Hall, Reference Hall1858, by original designation.
Included species
Cusacrinus arnoldi (Wachsmuth and Springer in Miller, Reference Miller1889); C. asperrimus (Meek and Worthen, Reference Meek and Worthen1870); C. brushi n. sp., C. chloris (Hall, Reference Hall1861a); C. coelatus (Hall, Reference Hall1858); C. daphne (Hall, Reference Hall1863); C. denticulatus (Wachsmuth and Springer, Reference Wachsmuth and Springer1897); C. ectypus (Meek and Worthen, Reference Meek and Worthen1870); C. kuenzii (Laudon, Parks, and Spreng, Reference Laudon, Parks and Spreng1952); C. limabrachiatus (Hall, Reference Hall1861a); C. longus (Meek and Worthen, Reference Meek and Worthen1870); C. nodobrachiatus (Wachsmuth and Springer in Miller, Reference Miller1889); C. ornatissimus (Wachsmuth and Springer in Miller, Reference Miller1897); C. penicillus (Meek and Worthen, Reference Meek and Worthen1870); C. sampsoni (Miller and Gurley, Reference Miller and Gurley1896b); C. sobrinus (Miller and Gurley, Reference Miller and Gurley1896b); C. spectabilis (Miller and Gurley, Reference Miller and Gurley1896b); C. spinotentaculus (Hall, Reference Hall1859); C. subscitulus (Miller and Gurley, Reference Miller and Gurley1896b); C. tenuisculptus (McChesney, Reference McChesney1860); C. thetis (Hall, Reference Hall1861b); C. tuberculosus (Wachsmuth and Springer, Reference Wachsmuth and Springer1897); and C. viaticus (White, Reference White1874).
Diagnosis
Basal circlet high or low; radial circlet high; one fixed secundibrachial; secundibrachitaxis highest brachitaxis in vertical wall of calyx; four ranges in regular interray; six ranges in posterior interray; interrays in contact with tegmen; plating in proximal interrays 1–2; fixed intrabrachials between half-rays present; arms weakly grouped; arm lobes absent; tegmen lower than or same height as calyx; many medium-sized plates on tegmen; anal tube central (modified from Rhenberg et al., Reference Rhenberg, Ausich and Kammer2015).
Occurrence
Mississippian (Tournaisian) of North America.
Remarks
Characters of Cusacrinus brushi n. sp. are consistent with those used to define the genus Cusacrinus in Rhenberg et al. (Reference Rhenberg, Ausich and Kammer2015).
Cusacrinus brushi new species
Figures 2.5, 3, 4.6
Type
Holotype: CMNH 18015a.
Diagnosis
Calyx shape low bowl, radial plates relatively high, stellate ray plate sculpturing (only one ridge to adjoining plates), median ray ridges present, two fixed primibrachials, one fixed secundibrachial, quartibrachials highest fixed brachials, stellate or nodose sculpturing on fixed interradial plates, fixed intraradial plates present, regular interray plates in contact with tegmen, ~6 free arms in each ray, and short spines on pinnulars (note: nature of the connection between the CD interray and the tegmen and the shape of the tegmen are unknown).
Occurrence
Mississippian (Tournaisian) Wooster Shale Member, Cuyahoga Formation at the abandoned Medal Brick and Tile Quarry, Wooster, Wayne County, Ohio, and along Shade Creek in Wayne County, Ohio.
Description
Crown large. Calyx large, cone shaped without a basal concavity. Calyx plate sculpturing prominent but highly variable (Fig. 3). Basal circlet ~10% of calyx height; basal plates presumably three, visible in lateral view but poorly preserved. Radial circlet ~15% of calyx height, presumably in lateral contact in all interrays except the CD interray; radial plates large, presumably five, hexagonal, approximately as wide as high. Radial plate sculpturing prominent stellate ridges connecting to adjoining plates and forming the beginning of ray ridges (Fig. 3.2).

Figure 3. Cusacrinus brushi n. sp., CMNH 18015. (1) Bed containing holotype that is completely replaced by siderite; note three crinoid specimens on slab, CMNH 18015a, holotype, large specimen on slab; CMNH 18015b, a Platycrinites s.l. on the left side of the calyx, CMNH 18015c, a Camerata indeterminate on the right side of the slab; a Platyceras gastropod mold is also above the calyx. (2) CMNH 18015a: enlargement of holotype, note fixed interradial and intraradial plates and variable plate sculpturing in interradial plates and short spines on pinnulars; Platycrinites s.l. on the left side of the calyx, a Platyceras gastropod mold above the calyx. Scale bar represents 10.0 mm in (1) and 5.0 mm in (2). Specimen in (2) coated with ammonium chloride sublimate for photography.
Regular interrays in narrow contact with tegmen, interradial plates large proximally and small distally, mostly hexagonal. First interradial plate slightly higher than wide with subtle radiating ridges (Fig. 3.2). Regular interray plating 1-2-3-3-4-2 (completely known on only one interray); plates in second range with subtle radiating ridges, plates in higher ranges slightly concave, with or without a low central node. CD interray not known.
Fixed brachials through at least the second or third quartibrachial, ~75% of calyx height; in each ray two primibrachials, one secundibrachial, two tertibrachials if branched or more if unbranched, 2 or more quartibrachials if present. Primibrachials through tertibrachials dominated by medial ray ridge; primibrachials through tertibrachials rectilinear uniserial, fixed quartibrachials weakly cuneate uniserial. Fixed intrabrachials medial between fixed secundibrachials and tertibrachials and between tertibrachials and quartibrachials in half-rays (Fig. 3.1, 3.2).
Tegmen unknown.
Free arms 30–40, high, atomous. Brachials chisel biserial after the third quartibrachial and deeper than wide. Pinnules long; pinnulars with spine projecting obliquely upward and more or less perpendicular to the pinnule attitude if arms closed.
Column circular with circular lumen. Other details unknown (Fig. 3.1, 3.2).
Etymology
This species recognizes Professor Nigel Brush, Ashland University and The College of Wooster, for his long geological and archaeological teaching career and his field work that has advanced our understanding of the Wooster Shale Member and its fossils.
Additional material
Three additional specimens are also assigned to this species: CMNH 4874a, CMNH 4874b, and CMNH 18014.
Measurements
CMNH 18015a: CrH, 112.0*; CaH, 42.0*, ACH, 15.0; CMNH 4874a: CaH, 36.0*; CaW, 57.0*; ColH, 61.0*.
Remarks
Cusacrinus has wide variability in character states (Supplementary Table 2). Cusacrinus brushi n. sp. is similar to a group of species that share the following character states: median ray ridges, two fixed primibrachials, one fixed secundibrachial, quartibrachials the highest fixed brachials in the calyx, regular interrays in contact with the tegmen, and ~6 free arms per ray. These species include Cusacrinus coelatus (lower Burlington Formation), Cusacrinus daphne (Meadville Shale Member, Cuyahoga Formation), Cusacrinus limabrachiatus (lower Burlington Formation), possibly Cusacrinus spectabilis (unspecified position in the Burlington Formation), and Cusacrinus spinotentaculus (lower Burlington Formation). These five species differ from Cusacrinus brushi n. sp. in a variety of ways. Cusacrinus coelatus has a low cone-shaped calyx, relatively low radial plates, stellate and multistellate ray-plate sculpturing (where stellate refers to those with a single ridge connecting to like ridges of adjoining plates and multistellate is the character state in which more than one ridge connects to like ridges to each adjoining plate), stellate sculpturing on interradial plates, fixed intraradial plates absent, regular interrays may or may not be in contact with the tegmen, the posterior interray is in relatively narrow contact with the tegmen, and the tegmen is a low cone shape (the presence or absence of spines on pinnulars is unknown). Cusacrinus daphne has a low cone-shaped calyx, relatively low radial plates, multistellate ray plate sculpturing on ray plates, multistellate plate sculpturing on interradial plates, fixed intraradial plates absent, regular interrays in contact with tegmen, and short spines on pinnulars (tegmen shape is unknown). Cusacrinus limabrachiatus has a low cone-shaped calyx, relatively low radial plates, stellate ray plate sculpturing, stellate plate sculpturing on fixed interradial plates, fixed intraradial plates absent, regular interrays in contact with tegmen, and short spines on pinnulars (tegmen shape is unknown). Cusacrinus spectabilis has a medium cone-shaped calyx, relatively high radial plates, smooth ray plate sculpturing, smooth sculpturing on interradial plates, and fixed intrabrachial plates absent (nature of the contact between the regular interrays, tegmen shape, and presence or absence of spines on pinnulars unknown). Cusacrinus spinotentaculus has a low cone- to urn-shaped calyx, relatively low radial plates, stellate or multistellate ray plate sculpturing, stellate sculpturing on interradial plates, fixed intraradial plates absent, regular interrays in contact with the tegmen, and a low cone shape (the presence or absence of spines on pinnulars could not be confirmed despite the species name “spinotentaculus”). In contrast, Cusacrinus brushi n. sp. has a low bowl-shaped calyx, relatively high radial plates, stellate ray plate sculpturing, stellate or nodose sculpturing on interradial plates, fixed intrabrachial plates present, regular interrays in contact with tegmen, and spines present on pinnulars (tegmen shape unknown).
Most species of Cusacrinus occur in Tournaisian carbonate settings (e.g., the Burlington Formation and Chouteau Limestone of Illinois, Iowa, and Missouri; the Maynes Creek Formation of Iowa; the Lake Valley Formation in New Mexico; the Henderson Canyon Formation of Utah; the Anchor Limestone of Nevada; the Lodgepole Formation of Montana; and the Banff Formation of Alberta, Canada) (see Webster, Reference Webster2014). The only Cusacrinus species known from siliciclastic-dominated facies are from the Cuyahoga Formation (Tournaisian) of Ohio: Cusacrinus daphne from the Meadville Shale Member and Cusacrinus brushi n. sp. from the Wooster Shale Member. These are distinct species, as noted above.
Two very poorly preserved camerate specimens are assigned to Cusacrinus brushi n. sp. CMNH 4874a is the proximal half of a calyx with ~60 mm of column attached (Fig. 4.6.). A smaller specimen (CMNH 4874b) is only the proximal portion a calyx. Both are preserved in dark gray shale and are similar to Cusacrinus brushi n. sp. by having large calyx plates. However, the preservation is insufficient to identify these specimens with confidence. CMNH 18014 is also assigned to Cusacrinus brushi n. sp. based on the sculpturing of fixed brachials. This specimen is a poorly exposed calyx with parts of one arm visible (Fig. 2.5). Also, this specimen has a Platyceras Conrad, Reference Conrad1840, gastropod attached to the tegmen. Curiously, the only other specimen of Cusacrinus brushi n. sp. with arms preserved (CMNH 18015a) also has a Platyceras gastropod attached (Fig. 3). Platyceratids are not preserved on other crinoids from this fauna.
Superfamily Carpocrinacea de Koninck and Le Hon, Reference de Koninck and Le Hon1854
Family Coelocrinidae Bather, Reference Bather1899
Genus Agaricocrinus Hall, Reference Hall1858
Type species
Agaricocrinus tuberosus Hall, Reference Hall1858.
Included species
Agaricocrinus americanus (Roemer, Reference Roemer and Brown1854); A. bellatrema Hall, Reference Hall1861a; A. bellatrema major Wachsmuth and Springer, Reference Wachsmuth and Springer1897; A. blairi Miller, Reference Miller1892b; A. brevis (Hall, Reference Hall1858); A. bullatus Hall, Reference Hall1858; A. conicus Wachsmuth and Springer, Reference Wachsmuth and Springer1897; A. convexus (Hall, Reference Hall1859); A. coreyi Lyon and Casseday, Reference Lyon and Casseday1860; A. crassus Wetherby, Reference Wetherby1881; A.? depressus (Casseday and Lyon, Reference Casseday and Lyon1862); A. excavatus Hall, Reference Hall1861b; A. fiscellus (Hall, Reference Hall1861a); A. geometricus (Hall, Reference Hall1859); A. gracilis Meek and Worthen, Reference Meek and Worthen1861; A. hodgsoni Miller and Gurley, Reference Miller and Gurley1896a; A. illinoisensis Miller and Gurley, Reference Miller and Gurley1896a; A. inflatus Hall, Reference Hall1861b; A. iowensis Miller and Gurley, Reference Miller and Gurley1897; A. jerseyensis (Worthen, Reference Worthen1890); A. louisianensis Rowley, Reference Rowley1900; A. montgomeryensis Peck and Keyte, Reference Peck, Keyte and Branson1938; A. murphyi n. sp.; A. nodosus Meek and Worthen, Reference Meek and Worthen1870; A. nodulosus Worthen in Miller, Reference Miller1889; A. nodulosus macadamsi Worthen in Miller, Reference Miller1889; A. planoconvexus Hall, Reference Hall1861b; A. praecursor Rowley, Reference Rowley1902b; A. pyramidatus (Hall, Reference Hall1858); A. sampsoni Miller, Reference Miller1892b; A. spendens Miller and Gurley, Reference Miller and Gurley1890; A. stellatus (Hall, Reference Hall1858); A. tuberosus Hall, Reference Hall1858; A. whitfieldi Hall, Reference Hall1858; and A. wortheni Hall, Reference Hall1858.
Occurrence
Mississippian (Tournaisian to Viséan); United States.
Agaricocrinus murphyi new species
Figure 4.7, 4.8
Types
Holotype: OSU 55204.
Diagnosis
Thecal size small, flat cone-shaped aboral cup, pentalobate calyx outline, narrow and small basal concavity, only basal plates in basal concavity, nodose outer surface of radial plate, small plates in the CD interray, convex outer surface of primibrachials, convex outer surface of first interradial plates, small plates in CD interray, small tegmen plates, convex or nodose fixed ambulacral cover plates, very nodose to spinose posterior primary peristomial cover plate, protuberant anal region, anus on side of tegmen, arm facets as wide as high, free arm facets project laterally, and 12 or more arms (tegmen shape, outer surface of non-CD interray primary peristomial cover plates unknown).
Occurrence
Wooster Shale Member, Cuyahoga Formation (Mississippian, Tournaisian) at abandoned shale pit ~1.5 miles south of New London, east side of Highway 60, Ruggles Township, Ashland County, Ohio.
Description
Calyx small, flat cone shape with narrow, shallow basal concavity (Fig. 4.7). Calyx plates modestly to very convex, with pustulose plate sculpturing, calyx plate triple junctions depressed (Fig. 4.7). Basal circlet confined entirely to the basal concavity. Radial circlet forms base of calyx, interrupted in CD interray. Radial plates five, heptagonal, wider that high, strongly convex (Fig. 4.7).
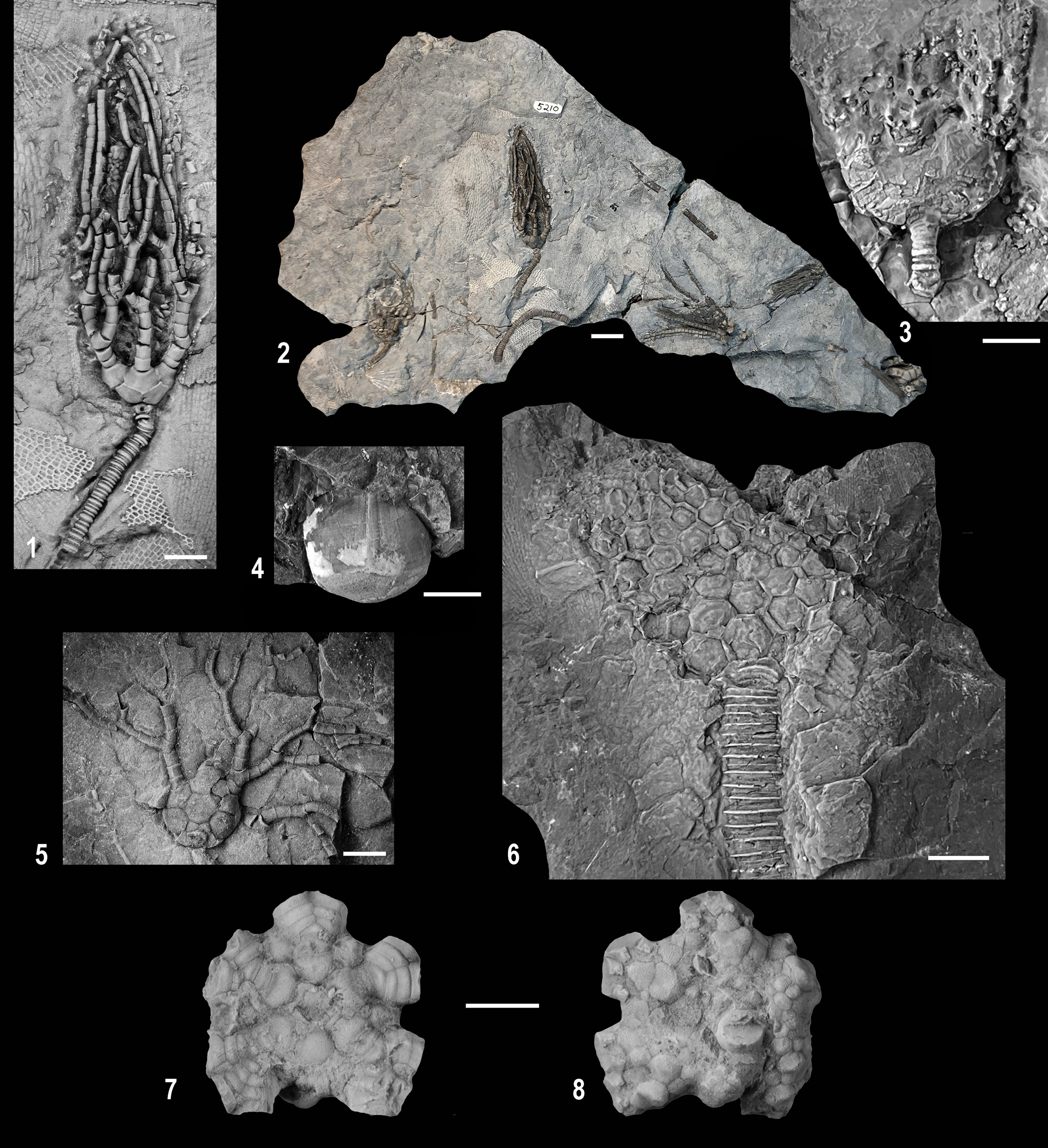
Figure 4. Wooster Shale Member crinoids. (1, 2, 5) Cyathocrinites simplex (1, 2) CMNH 5210; (1) CMNH 5210a, well-preserved specimen with partial arms and column; (2) bedding surface with well-preserved specimen shown in (1) with partial arms and column; (5) CMNH 5211a, oblique lateral view of a partially preserved specimen in the CD interray and three partial arms spread out illustrating arm branching. (3, 4) Platycrinites s.l. sp. (3) CMNH 18015b, poorly preserved, partly collapsed specimen with very poorly preserved brachials above and proximal column below; (4) CMNH 18016, internal mold of calyx. (6) Cusacrinus brushi n. sp., CMNH 4874a, internal mold of calyx and proximal column. (7, 8) Agaricocrinus murphyi n. sp., holotype, OSU 55204; (7) aboral view; note flat basal of calyx with basal plates in a concavity and very convex radial plates; (8) oral view of poorly preserved tegmen. Scale bar represents 5.0 mm in (1–6) and 10.0 mm in (7, 8). Specimens in (1, 3–8) coated with ammonium chloride sublimate for photography.
Regular interrays in contact with tegmen; first interradial higher than wide, smaller than radial plates and larger than primibrachials. Regular interray plating 1-?. Primanal hexagonal, wider than high, approximately the same size as radial plates, interrupts the radial circlet; plating in CD interray incompletely known; CD interray in contact with tegmen.
In the A, B, and E rays, two primibrachials and two to three secundibrachials fixed, in D and E rays two primibrachials, one to three secundibrachials, and three tertibrachials fixed with secundaxil on CD interray side of C and D rays. Fixed brachials convex and uniserial.
Tegmen multiplated, pustulose plate sculpturing, large plates around outer perimeter and decreasing plate size toward center of tegmen with the exception of one very large nodose to spinose tegmen plate peripherally located in each ray (Fig. 4.8). Tegmen connection to calyx broad with numerous plates (plating pattern unknown). Anal opening on a small, loosely plated protuberance.
Twelve free arms, two from A, B, and C rays and three from C and D rays.
Free arms and column unknown.
Etymology
This name recognizes the late James L. Murphy, who collected the holotype of this new species as well as other Wooster Shale Member crinoids described in this paper. Now deceased, James L. Murphy was a librarian at The Ohio State University and an avocational paleontologist and archaeologist. His paleontological collections are housed in the Orton Geological Museum, The Ohio State University.
Measurements
OSU 55204 (holotype): CaH, 7.5*: CaW, 14.7*.
Remarks
A single Wooster Shale Member specimen (OSU 55204) is assigned to Agaricocrinus murphyi n. sp. This is a small individual with a flat calyx and a collapsed tegmen. Numerous species are assigned to Agaricocrinus (Webster, Reference Webster2014), which is a characteristic and common crinoid in the Tournaisian and early Viséan of North America. As presently understood, 33 species (and two subspecies) are assigned to Agaricocrinus. The genus as a whole has not had a recent comprehensive review, and valid species concepts undoubtedly require revisions. However, such a review is beyond the scope of the present paper.
One criterion by which to group Agaricocrinus species is by the thecal outline (from either an oral or aboral perspective). Most species have a pentalobate outline, but some have either a pentagonal or circular outline (Supplemental Table 3). Agaricocrinus species with a pentalobate outline can be further differentiated by the width and depth of the basal concavity and by the plate circlet(s) that are present in the basal concavity. Agaricocrinus montgomeryensis Peck and Keyte, Reference Peck, Keyte and Branson1938; A. sampsoni Miller, Reference Miller1892b; and A. murphyi n. sp. all have a narrow and shallow basal concavity and only basal plates in the basal concavity. Further, these three species are all from lower Tournaisian strata. These three species can be differentiated because A. montgomeryensis has a very low cone- to urn-shaped calyx, convex radial plates, large plates in the posterior interray, large tegmen plates, spinose primary peristomial cover plates, spinose CD interray primary peristomial cover plate, arm facets wider than high, and ten free arms; and A. sampsoni has convex radial plates and ten free arms (other aspects of its morphology are unknown). In contrast, A. murphyi n. sp. has a flat cone-shaped calyx, nodose radial plates, small plates in the posterior interray, small tegmen plates, very convex primary peristomial cover plates, very nodose CD interray primary peristomial cover plate, arm facets as wide as high, and probably twelve free arms or more (Supplemental Table 3).
Suborder Glyptocrinina Moore, Reference Moore, Moore, Lalicker and Fischer1952
Superfamily Platycrinitoidea Austin and Austin, Reference Austin and Austin1842
Family Platycrinitidae Austin and Austin, Reference Austin and Austin1842
Genus Platycrinites Miller, Reference Miller1821
Type species
Platycrinites laevis Miller, Reference Miller1821.
Remarks
Platycrinites was described by Miller (Reference Miller1821) in the same publication in which he defined the Crinoidea. Consequently, this genus name has been used widely. As discussed in Ausich and Kammer (Reference Ausich and Kammer2009), the helically twisted column historically used as a diagnostic character for Platycrinites is a diagnostic character for the Platycrinitidae, and genera are largely differentiated by different non-column characters. Key among these is knowledge of the tegmen: is an anal tube or a simple anal opening present on the tegmen? Unfortunately, this key character is unknown on many species that were historically assigned to Platycrinites. Species should be assigned to Platycrinites sensu lato if the morphology of the tegmen and/or other generic diagnostic characters are unknown (Ausich and Kammer, Reference Ausich and Kammer2009). Accordingly, the Wooster Shale Member platycrinitid is assigned to Platycrinites s.l. sp.
Platycrinites s.l. sp.
Figures 3.2, 4.3, 4.4
Occurrence
Mississippian (Tournaisian) Wooster Shale Member, Cuyahoga Formation at the abandoned Medal Brick and Tile Quarry, Wooster, Wayne County, Ohio.
Description
Aboral cup medium bowl shape, plates thin. Basal circlet convex, ~20% of calyx height, sculpturing concentric ridges around entire basal circlet and centered on column facet; basal plates five, pentagonal. Radial circlet ~80% of calyx height; radial plates five, hexagonal, higher than wide, diagonal ridge sculpturing possible on radial plates. No fixed interradial plates or fixed brachials. CD interray, tegmen, arms, and column unknown.
Materials
CMNH 18015b, CMNH 18016.
Measurements
CMNH 18016: AcH, 11.0*; CaW, 12.2.
Remarks
Two incomplete and poorly preserved specimens of Platycrinites are known from the Wooster Shale Member. CMHN 18016 is preserved as a partial internal mold. Only the basal circlet and two partial radial plates are visible on this specimen (Fig. 4.4). The basal circlet appears to have low concentric ridge sculpturing, and the radial plates are upright. CMNH 18015b is a crushed aboral cup with a few disarticulated brachials and a few proximal columnals preserved (Fig. 4.3).
As preservation allows, Wooster Shale Member specimens are different from all Platycrinites species known from the Meadville Shale Member of the Cuyahoga Formation (Ausich and Roeser, Reference Ausich and Roeser2012) (i.e., P. s.l. burkei Ausich and Roeser, Reference Ausich and Roeser2012; P. s.l. contritus [Hall, Reference Hall1863]; P. s.l. graphicus [Hall, Reference Hall1863]; and P. s.l. lodensis [Hall and Whitfield, Reference Hall and Whitfield1875]). Wooster Shale Member specimens are too incompletely known to place it in a species with confidence; therefore, it is left in open nomenclature as Platycrinites s.l. sp.
Subclass Pentacrinoidea Jaekel,Reference Jaekel1894
Infraclass Inadunata Wachsmuth and Springer, Reference Wachsmuth1885
Parvclass Cladida Moore and Laudon, Reference Moore and Laudon1943
Magorder Eucladida Wright, Reference Wright2017
Superorder Cyathoformes Wright et al., Reference Wright, Ausich, Cole, Rhenberg and Peter2017
Cyathoformes incertae sedis: “Cyathocrinida” Bather, Reference Bather1899
Family Cyathocrinidae Bassler, Reference Bassler1938
Genus Cyathocrinites Miller, Reference Miller1821
Included species
Cyathocrinites abbreviatus Miller, Reference Miller1821; C. asperrimus (Springer, Reference Springer1911); C. barrisi (Hall, Reference Hall1861a); C. barydactylus (Wachsmuth and Springer, Reference Wachsmuth and Springer1878); C. bursa (Phillips, Reference Phillips1836); C. calcaratus (Phillips, Reference Phillips1836); C. chouteauensis (Miller and Gurley, Reference Miller and Gurley1896a); C. decaphyllus Roemer, Reference Roemer1843; C. distortus (Gilbertson in Phillips, Reference Phillips1836); C. dubius (Münster, Reference Münster1840); C. elongatus (Knod, Reference Knod1908); C. faberi (Miller and Gurley, Reference Miller and Gurley1896b); C. farleyi (Meek and Worthen, Reference Meek and Worthen1866); C. fischeri (Spandel, Reference Spandel1899); C.? fonei Donovan et al., Reference Donovan, Widdison, Lewis and Fearnhead2010; C. formosus (Rowley, Reference Rowley1905); C. foveolatus (Eichwald, Reference Eichwald1856); C. gilesi (Wachsmuth and Springer, Reference Wachsmuth and Springer1878); C. glenni Ausich and Lane, Reference Ausich and Lane1982; C. globosus (Troost in Wood, Reference Wood1909); C. gosae (Roemer, Reference Roemer1866); C. granulatus (Münster, Reference Münster1839); C. harrodi (Wachsmuth and Springer, Reference Wachsmuth and Springer1880); C. ignotus (Trenkner, Reference Trenkner1868); C.? inaequidactylus (M'Coy, Reference M'Coy1844); C. iowensis (Owen and Shumard, Reference Owen and Shumard1850); C. irregularis (Trenkner, Reference Trenkner1868); C. kelloggi (White, Reference White1862); C. lamellosus (White, Reference White1863); C. macadamsi (Miller and Gurley, Reference Miller and Gurley1895); C. mammillaris (Phillips, Reference Phillips1836); C. marshallensis (Worthen, Reference Worthen1882); C. milleri (M'Coy, Reference M'Coy1844); C. multibrachiatus (Lyon and Casseday, Reference Lyon and Casseday1859); C. multibrachiatus squarrosa (Hall, Reference Hall1872); C. patulosus (Wright, Reference Wright1935); C. planus Miller, Reference Miller1821; C.? radiatus (Austin and Austin, Reference Austin and Austin1843) (non Eichwald, Reference Eichwald1856); C. radiatus (Eichwald, Reference Eichwald1856) (non Austin and Austin, Reference Austin and Austin1843); C.? ramosus (Schlotheim, Reference Schlotheim1817); C. rarus (Lyon, Reference Lyon1869); C. rigidus (White, Reference White1862); C. rockfordensis (Thomas, Reference Thomas1924); C. saffordi (Meek and Worthen, Reference Meek and Worthen1860); C. sampsoni (Miller, Reference Miller1891); C. simplex Kammer and Roeser, Reference Kammer and Roeser2012; C. sphaericus (Steininger, Reference Steininger1849); C. stubblefieldi Wright, J., Reference Wright1952; C. subtuberculatus (Roemer, Reference Roemer1866); C. tenuidactylus Meek and Worthen, Reference Meek and Worthen1868; C. teres (Münster, 1840); C. tricarinatus Roemer, Reference Roemer1843; C. tuberculatus (Roemer, 1850) (non Miller, Reference Miller1821); C. turbinatus (Weller, Reference Weller1900); C. variabilis (Phillips, Reference Phillips1841); and C. virgalensis (Waagen, Reference Waagen1887).
Cyathocrinites simplex Kammer and Roeser, Reference Kammer and Roeser2012
Figure 4.1, 4.2, 4.5
Type
Holotype: CMCIP 46159; paratypes: CMCIP 5718-20, and CMCIP 5956-10.
Diagnosis
Cyathocrinites with a low bowl-shaped aboral cup; infrabasals visible in lateral view, thin plates; smooth sculpturing; and smooth, elongate brachials (modified from Kammer and Roeser, Reference Kammer and Roeser2012).
Occurrence
Meadville Shale and Wooster Shale members of the Cuyahoga Formation in northeastern Ohio; Mississippian (Tournaisian). New occurrences in the Wooster Shale member are from Wooster, Ohio, in the abandoned Medal Brick and Tile Quarry, Wooster, Wayne County, Ohio.
Description
Crown small in size, subcylindrical. Aboral cup low bowl shape, plates thin with smooth sculpturing other than very subtle radiating folding (Fig. 4.1). Infrabasal circlet ~6.8% of aboral cup height; presumably five, equal in size, as known. Basal circlet ~38.6% of aboral cup height; basal plates five, hexagonal, slightly larger than radial plates, ~1.3 times wider than high. Radial circlet ~54.5% of aboral cup height; radial plates five, heptagonal, wider than high. Radial facet angustary (Fig. 4.1), ~42% of distal radial plate width, semicircular, declivate; radial facet topography unknown.
CD interray with only the radianal within aboral cup (Fig. 4.5). Anal sac presumably cylindrical, ~70% of height of arms, distal anal sac with small plates, distalmost portion of sac surrounded by a ring of nodose plates.
Arms slender, branch three or four times with poor isotomy. Primibrachial dimensions variable, higher than wide or wider than high. Third to fifth primibrachial axillary (Fig. 4.1, 4.5), primaxil as wide as high. Secundibrachial higher than wide, third to fifth secundibrachial axillary. All brachials rectangular uniserial, sharply convex, straight sides. Free arms progressively smaller above each axillary, free arm shape at axillaries more of a U-shape than a V-shape (the latter of which is more typical in crinoids).
Proximal column circular, heteromorphic; columnar index N212, holomeric. Nodals with variable, small nodes around latus; holdfast unknown.
Material
Wooster Shale Member specimens CMNH 5210a–CMNH 5110c, CMNH 5211a, and CMNH 5211b.
Measurements
CMNH 5210a: CrH, 41.5; ACH, 5.4; ACW, 8.5; CoH, 18.0*.
Remarks
Cyathocrinites is a geographically and temporally widespread genus. Cyathocrinites belongs to the primitive cladid clade (sensu Kammer et al., Reference Kammer, Baumiller and Ausich1997, Reference Kammer, Baumiller and Ausich1998) or the Cyathoformes incertae sedis: “Cyathocrinida” clade (sensu Wright et al., Reference Wright, Ausich, Cole, Rhenberg and Peter2017). Cyathocrinites simplex was first described from the Meadville Shale Member of the Cuyahoga Formation, and it also occurs in the Wooster Shale Member of the Cuyahoga Formation. CMNH 5210a is an excellently preserved specimen and adds to our understanding of this species. Kammer and Roeser (Reference Kammer and Roeser2012, p. 473) outlined the species characters that differentiate C. simplex from other lower Tournaisian Cyathocrinites species.
Superorder Flexibilia Zittel, Reference Zittel1895
Order Taxocrinida Springer, Reference Springer, Zittel and Eastman1913
Family Taxocrinidae Angelin, Reference Angelin1878
Genus Taxocrinus Phillips in Morris, Reference Morris1843
Type species
Cyathocrinus? macrodactylus Phillips, Reference Phillips1841, by subsequent designation.
Other species
Taxocrinus anomalus Waters et al., Reference Waters, Maples, Lane, Marcus, Liao, Liu, Hou and Wang2003; T. belgicus Springer, Reference Springer1920; T. bellmanensis Wright, Reference Wright1954; T. colletti White, Reference White1881; T. communis (Hall, Reference Hall1863); T. coplowensis Wright, Reference Wright1946; T. delabolei Wright, Reference Wright1937; T. giddingsi (Hall, Reference Hall1858); T. granulatus Salter, Reference Salter1873; T. hibernicus (Wright, Reference Wright1934); T. hollandi Laudon and Beane, Reference Laudon and Beane1937; T. huntsvillae Springer, Reference Springer1920; T. intermedius Wachsmuth and Springer, Reference Wachsmuth and Springer1888; T. interscapularis Hall, Reference Hall1858; T. juvenis (Hall, Reference Hall1861a); T. kellogi (Hall, Reference Hall1863); T. lobatus (Hall, Reference Hall1862); T. macrodactylus (Phillips, Reference Phillips1841); T. nobilis (Phillips, Reference Phillips1836); T. ornatus Springer, Reference Springer1920; T. praestans Springer, Reference Springer1920; T. priscus Steininger, Reference Steininger1853; T. pustulosus Springer, Reference Springer1920; T. ramulosus (Hall, Reference Hall1859); T. shumardianus (Hall, Reference Hall1858); T. strimplei Knox and Kendrick, Reference Knox and Kendrick1987; T. stultus Whidborne, Reference Whidborne1896; T. telleri Springer, Reference Springer1920; T. ungula Miller and Gurley, Reference Miller and Gurley1896a; and T. whitfieldi (Hall, Reference Hall1858).
Occurrence
Devonian (Givetian) to Mississippian (Serpukhovian); Belgium, China, Germany, Ireland, United Kingdom, United States.
Taxocrinus sp.
Figure 5.5
Occurrence
Mississippian (Tournaisian) Wooster Shale Member, Cuyahoga Formation in the abandoned Medal Brick and Tile Quarry, Wooster, Wayne County, Ohio.

Figure 5. Eucladid crinoids from the Wooster Shale Member. (1) Decadocrinus laevis n. sp., CMNH 4873, holotype, lateral view of partially disarticulated crown with proximal column attached. (2, 3) Decadocrinus inordinatus n. sp.; (2) OSU 53548, holotype, CD-interray view of partial crown with proximal column attached; (3) CMNH 5214a, paratype, lateral view of a partial crown with proximal column attached. (4) Eucladid indeterminate, CMNH 5213, juvenile specimen with a partial crown and proximal portion of the column preserved. (5) Taxocrinus sp., CMNH 5215, partial crown with column, note the striking contrast between columnals of the proxistele versus the mesistele. Scale bar represents 5.0 mm in all. All specimens coated with ammonium chloride sublimate for photography.
Description
Crown medium in size. Aboral cup low cone shaped (Fig. 5.5), height to width ratio ~0.6; basal concavity rimmed by ridge on basal plates, plates gently convex, smooth plate sculpturing.
Infrabasal circlet confined to basal concavity, not visible in lateral view. Basal circlet ~37% of aboral cup height, ridge around the basal circlet at the proximal lateral edge of aboral cup; basal plates presumably five, hexagonal, ~1.6 times wider than high, much smaller than radial plates. Radial circlet ~63% of aboral cup height; radial plates presumably five, pentagonal, slightly higher than wide. Radial facets plenary, indentation on margin of radial facet for patelloid process of first primibrachial.
CD interray and anal sac unknown.
Arms robust, branch two times as known with poor isotomy (Fig. 5.5). Brachials rectangular uniserial, convex, straight sides, wider than high, well-developed patelloid processes, smooth plate sculpturing.
Column circular in shape, holomeric, heteromorphic, distinct proxistele and mesistele. Proxistele homeomorphic, columnals ~10 times wider than high, latus convex; mesistele heteromorphic, distal portion of preserved mesistele with N212 pattern, nodals significantly larger than internodals (Fig. 5.5). Distal mesistele, dististele, and holdfast not known.
Materials
CMNH 5215.
Measurements
CMNH 5215: CrH, 35.9; ACH, 4.4; ACW, 8.9; CoH, 29.0*.
Remarks
A single, incomplete flexible crinoid is known from the Wooster Shale Member. This is a specimen with an aboral cup, most of one arm, a bit of a second arm, the proxistele, and the proximal portion of the mesistele (Fig. 5.5). This specimen may be a new species; but a full understanding of the arm branching pattern and posterior interray is needed to assign this specimen to a species.
Taxocrinus is a geographically widespread and temporally long-ranging Mississippian–Devonian flexible crinoid. Four species of Taxocrinus have been reported from the early Tournaisian of North America, including T. communis and T. kellogi from the Cuyahoga Shale Member of the Cuyahoga Formation and T. hollandi and T. intermedius from the Maynes Creek Formation. In addition, Dactylocrinus tardus (Hall, Reference Hall1862) is also known from the Meadville Shale Member of the Cuyahoga Formation. Dactylocrinus tardus has endotomous arm branching above the primaxil, which sharply contrasts with Taxocrinus, in general, and with Taxocrinus sp. from the Wooster Shale Member. Taxocrinus sp. is also distinct from the other four early Tournaisian species assigned to Taxocrinus. Taxocrinus communis differs by having first interradial plates sutured between first primibrachials and adjoining rays, and the proximal portion of the mesistele is homeomorphic with large columnals. In contrast, T. sp. from the Wooster Shale Member lacks an interradial plate between first primibrachials and has a heteromorphic proximal portion of the mesistele. Taxocrinus hollandi has infrabasal plates visible in lateral view, basal plates the largest plates in the aboral cup, narrow arms with brachials higher than wide, and an indistinct boundary between the proxistele and mesistele, whereas T. sp. from the Wooster Shale Member has infrabasals not visible in lateral view, radial plates the largest plates of the aboral cup, wide arms with brachials wider than high, and a distinct boundary between the proxistele and mesistele. Taxocrinus intermedius has a subspherical-shaped crown, infrabasals visible in lateral view, as many as four ranges of regular interradial plates between radials and fixed primibrachials of regular interrays, and intraradials between secundibrachials within a ray; whereas T. sp. presumably has an elongate crown, infrabasals are not visible in lateral view, and does not have either interradial or intraradial plates. Taxocrinus kellogi has infrabasal plates visible in lateral view, one interradial between first primibrachials of adjacent regular interrays, and short, stout spines on every axillary brachial; whereas T. sp. from the Wooster Shale does not have infrabasal plates visible in lateral view, lacks interradial plates, and lacks spines on axillary brachials.
The Wooster Shale Member Taxocrinus specimen (Fig. 5.5) is distinct from any known congener. However, the morphology of this specimen is insufficiently known to justify naming a new species, so this single specimen is left in open nomenclature as Taxocrinus sp.
Magnorder Eucladida Wright, Reference Wright2017
Cyathoformes incertae sedis: ‘Poteriocrinida’ Jaekel, Reference Jaekel1918
Family Decadocrinidae Bather, Reference Bather1890
Genus Decadocrinus Wachsmuth and Springer, Reference Wachsmuth and Springer1880
Type species
Poteriocrinus (Scaphocrinus) scalaris Meek and Worthen, Reference Meek and Worthen1870.
Included species
Decadocrinus aegina (Hall, Reference Hall1863); D. baumgardeneri Laudon and Beane, Reference Laudon and Beane1937; D. brazeauensis Laudon, Parks, and Spreng, Reference Laudon, Parks and Spreng1952; “D.” constrictus Lane, Waters, and Maples, Reference Lane, Waters and Maples1997; D. crassidactylus Laudon, Reference Laudon1936; D. clypeus Webster, Maples, and Yazdi, Reference Webster, Maples and Yazdi2007; D. decemnodosus Goldring, Reference Goldring1923; “D.” elongatus Lane, Waters, and Maples, Reference Lane, Waters and Maples1997; D.? exornatus Hauser, Reference Hauser1999; D. gregarious (Williams, Reference Williams1882); D. hughwingi Kesling, Reference Kesling1964; D. inordinatus n. sp.; D. insolens Goldring, Reference Goldring1923; D. kersadiouensis Le Menn, Reference Le Menn1985; D. killawogensis Goldring, Reference Goldring1923; D. laevis n. sp.; D. liriope Wachsmuth and Springer, Reference Wachsmuth and Springer1880; D. multinodosus Goldring, Reference Goldring1923; D. multinodosus var. serratobrachiatus Goldring, Reference Goldring1923; D. nereus (Hall, Reference Hall1862); D. oaktrovensis Webby, Reference Webby1961; D. ornatus Goldring, Reference Goldring1954; D. pachydactylus Laudon, Reference Laudon1936; D. penicilliformis (Worthen, Reference Worthen1882); D. regulatis Strimple, Reference Strimple1939; D. rugistriatus Goldring, Reference Goldring1923; “D.” rugosus Lane, Waters, and Maples, Reference Lane, Waters and Maples1997; D. scalaris (Meek and Worthen, Reference Meek and Worthen1870); D. spinobrachiatus Goldring, Reference Goldring1938; D. spinulifer Laudon, Reference Laudon1936; D. stewartae Kier, Reference Kier1952; D. tumidulus (Miller and Gurley, Reference Miller and Gurley1893); “D.” usitatus Lane, Waters, and Maples, Reference Lane, Waters and Maples1997; D. vintonensis Thomas, Reference Thomas1924; D. wrightae Goldring, Reference Goldring1954; and D. wrightae silicaensis Kesling, Reference Kesling1971.
Occurrence
Devonian to Pennsylvanian; Canada, China, France, Iran, United Kingdom, United States.
Remarks
Generic assignment of Wooster Shale Member eucladids is problematic. These taxa may represent a new genus, but our lack of knowledge of key morphological features (e.g., the nature of arm branching in the A ray) precludes erecting a new genus for these species. These crinoids are most closely associated with Decadocrinus and Pachylocrinus Wachsmuth and Springer, Reference Wachsmuth and Springer1880 (see Kammer and Ausich, Reference Kammer and Ausich1993, table 1); however, both Decadocrinus and Pachylocrinus have historically served as catch-all genera for Devonian and Mississippian eucladids with two primibrachials (T.W. Kammer, personal communication, 2023). Further, as presently understood (see Webster, Reference Webster2014), both of these genera are exceedingly long-ranging (Devonian to Pennsylvanian) and morphologically diverse. Kammer and Ausich (Reference Kammer and Ausich1993) tackled this problem for some early Viséan species, but further work is necessary. The type species of Decadocrinus and Pachylocrinus, D. scalaris and P. aequalis, respectively, are strikingly distinct, but species currently assigned to each genus blur the distinctions of the type species. A systematic review of the genus concepts of eucladids with two primibrachials and assignment of species is much needed, but such an analysis is beyond the scope of the present study.
As noted above, the type specimens of Decadocrinus and Pachylocrinus are distinct, but neither closely resembles the two Wooster Shale Member eucladids under consideration herein. However, it is our judgement that these two Wooster Shale Member species more closely resemble the collection of species currently recognized in Decadocrinus than those assigned to Pachylocrinus. Therefore, for this study, we assign these two new species to Decadocrinus with full recognition that they may be reassigned to a new genus after further systematic study. Assignment of these species to Decadocrinus is considered tentative pending further study.
The two Wooster Shale Member species of Decadocrinus share the following characteristics: infrabasals visible in lateral view, number of primibrachials, number of secundibrachials if branched, free arm branching, shape of the brachials, shape of pinnules, and column shape. As discussed below, they have contrasting aboral cup shape, aboral cup and brachial sculpturing, and shape of the radial plates. The Wooster Shale Member Decadocrinus species are compared to other current species in this genus in Supplemental Table 4.
Decadocrinus laevis new species
Figure 5.1
Type
Holotype: CMNH 4873.
Diagnosis
Low cone aboral cup shape; plate sculpturing smooth with scalloped plate margins; infrabasal plates visible in lateral view; radial plates ~1.25 times wider than high; radial facets plenary, declivate, crescentic; 12 or more total free arms; arm branching isotomous; second primibrachial axillary; third secundibrachial axillary if secundibrachitaxis branches; depression at midpoint along brachial-to-brachial sutures; brachials rectilinear uniserial; column shape pentalobate.
Occurrence
Wooster Shale Member, Cuyahoga Formation; Wooster, Wayne County, Ohio in the abandoned Medal Brick and Tile Quarry, Wooster, Wayne County, Ohio.
Description
Crown large in size. Aboral cup probably low cone shape (if uncompacted), height to width ratio of compressed aboral cup ~0.8; plates gently convex, smooth sculpturing, scalloped plate margins (Fig. 5.1).
Infrabasals presumably five and equal in size as known, visible in lateral view. Basal plates presumably five, hexagonal, smaller than radial plates, ~1.56 times wider than high. Radials presumably five, pentagonal, ~2.0 times wider than high. Radial facets plenary, crescentic, declivate, articular ridge across width of facet, an aboral ligament groove across most of the width of the facet.
CD interray plating slightly disarticulated, presumably three posterior plates in aboral cup interpreted as follows: radianal plate beneath and to the left of the C ray radial plate, anal X above to left of radianal and between C and D radial plates, and proximal part of right sac plate in articulation with the C radial to right and radianal beneath; above these plates a biseries of higher than wide plates lead to anal sac (Fig. 5.1).
Anal sac unknown.
Arms long, pinnulate, branch once or twice with poor isotomy; 12 or more total free arms. Primibrachials wider than high, primibrachial 2 axillary, primaxil wider than high. Secundibrachials wider than high, if secundibrachials branched secundibrachial 3 axillary. All brachials rectangular uniserial, gently convex aborally, straight sides, and a central depression along suture with adjacent brachials. Pinnules long, slender.
Proximal column pentalobate, holomeric, heteromorphic (N1) (Fig. 5.1). Lumen and holdfast unknown.
Etymology
The species name, laevis, means smooth (L., m.) and refers to the smooth aboral cup plate sculpturing.
Measurements
CMNH 4873 (holotype): CrH, 119.0; ACH, 11.0*; ACW, 15.0*; ColH, 7.0*.
Remarks
Decadocrinus laevis n. sp. is distinguished from D. inordinatus n. sp. because the former has a low cone-shaped aboral cup, smooth plate sculpturing with scalloped aboral cup margins, radial plates ~1.25 times wider than high, and a depression at the midpoint along brachial-to-brachial sutures. In contrast, D. inordinatus n. sp. has a low bowl-shaped aboral cup, irregularly reticulate plate sculpturing that approaches irregular ridges on radial plates, plate margins not scalloped, radial plates ~1.6 times wider than high, and irregularly reticulate plate sculpturing on brachials.
Comparison to other species of Decadocrinus is in Supplemental Table 4.
Decadocrinus inordinatus new species
Figure 5.2, 5.3
Type
Holotype: OSU 53548; paratypes: CMNH 5214a, CMNH 5214b.
Diagnosis
Low bowl aboral cup shape; plate sculpturing irregularly reticulate and approaches irregular ridges on radial plates; infrabasal plates visible in lateral view; radial plates ~1.6 times wider than high; radial facets plenary, declivate, crescentic; 12 or more total free arms; arm branching isotomous; second primibrachial axillary; third secundibrachial axillary if secundibrachitaxis branches; irregularly reticulate sculpturing on brachials; brachials rectilinear uniserial; column shape pentalobate.
Occurrence
Wooster Shale Member, Cuyahoga Formation; Wooster, Wayne County, Ohio, in the abandoned Medal Brick and Tile Quarry, Wooster, Wayne County, Ohio.
Description
Crown large in size (Fig. 5.2), aboral cup low bowl shaped, height to width ratio ~0.43, plates gently convex with irregularly reticulate sculpturing that on radial plates approaches irregular ridges projecting obliquely downward from rim of radial facets (Fig. 5.3).
Infrabasals circlet ~11% of aboral cup height, visible in lateral view; infrabasal plates presumably five. Basal circlet ~41% of aboral cup height; basal plates presumably five, hexagonal, smaller than radial plates ~1.5 times wider than high. Radial circlet ~48% of aboral cup height; radials presumably five, pentagonal, ~1.7 times wider than high. Radial facets plenary, slightly crescentic, radial facet topography unknown.
CD interray with three posterior plates in aboral cup, radianal below and to the left of the C radial plate, anal X above to the left of radianal and sutured to the D radial plate, right sac plate with proximal part in sutural contact with radianal below and C radial to the right (Fig. 5.2).
Anal sac unknown.
Arms long, slender, branch once or twice with poor isotomy; 12 or more total free arms. Primibrachials wider than high; if branched, primibrachial 2–3 axillary, primaxil wider than high. Secundibrachials wider than high; if branched, secundibrachials 3 or higher axillary. All brachials rectangular uniserial, convex aborally, straight sides, and plate sculpturing same as aboral cup plate sculpturing. Pinnules long, slender.
Proximal column pentalobate (Fig. 5.2), holomeric, heteromorphic, lumen pentalobate, holdfast unknown.
Etymology
The species name means irregular (Latin) and refers to the sculpturing on the aboral cup plates.
Measurements
OSU 53548 (holotype): CrH, 50.0*, ACH, 4.7: ACW, 14.0*; ColH, 3.0*. CMNH 5214a (paratype): CrH, 64.9*, ACH, 5.6: ACW, 14.3; ColH, 7.4*. CMNH 5214b (paratype): CrH, 47.0*.
Remarks
Decadocrinus inordinatus n. sp. is compared to D. laevis n. sp. in the discussion of D. laevis n. sp. and to all species currently assigned to Decadocrinus in Supplemental Table 4.
Eucladida indeterminate
Figure 5.4
Remarks
CMNH 5213 (Fig. 5.4) is a juvenile eucladid crinoid with a pentalobate column, infrabasal plates not visible, in lateral view, three or more primibrachials, and arms that probably branch two times. The presence of three primibrachials distinguishes this crinoid from the Wooster Shale Member Decadocrinus species, but the lack of other known characters precludes assigning this specimen to a genus or species, Thus, it is left in open nomenclature as Eucladida indeterminate.
Preservation
As described above, the Wooster Shale Member is predominantly dark gray shale with common siderite concretions and scattered concentrations of brachiopods, crinoids, mollusks, and bryozoans. The most prominent fossils on the outcrop are brachiopods (large spiriferinids and rhynchonellids), crinoid pluricolumnals, and platyceratid gastropods. Crinoids are variously preserved from completely disarticulated fragments to complete crowns with the proximal column attached. The most common crinoidal remains are individual columnals and lengths of pluricolumnals. If multiple pluricolumnals are preserved together, they are either randomly oriented or strongly aligned. Individual lengths of a pluricolumnal also may be broken into shorter “broken stick” column segments (Baumiller and Ausich, Reference Baumiller and Ausich1992), suggesting that these pluricolumnals laid exposed on the sea floor for a short time.
Crinoids are preserved in both the “shaving brush” and “starburst” trauma postures (Baumiller et al., Reference Baumiller, Gahn, Hess, Messing, Ausich and Webster2008; Messing et al., Reference Messing, Ausich and Meyer2021). Two of the five specimens of C. simplex are preserved in a partial starburst posture (e.g., Fig. 4.5), but the majority of other specimens are preserved in a well-defined shaving brush posture (e.g., Figs. 2.4, 4.1, 5.1). The occurrence of crinoids in these trauma postures is consistent with episodic high turbulence events, which is suggested by lenses of crinoidal packstone (some with shale rip-up clasts) in the Wooster Shale Member. This is consistent with a relatively shallow depositional environment between the fair-weather and storm wave bases (Clayton et al., Reference Clayton, Manger and Owens1998).
Individual crinoid specimens occur in several preservational modes, indicating a complex diagenetic history for these fossils. Perhaps the most common state of preservation is for crinoid plates to be preserved as external molds on outcrop bedding surfaces. In this condition, specimens simply disintegrate due to weathering, and collected specimens are very fragile. Crinoid plates are also commonly preserved with their original calcite. Specimens that retain calcite preservation of their plates are encased in shale (e.g., Figs. 2.3, 5.1, 5.2, 5.5).
Fossils in the Wooster Shale, including the crinoids, are commonly preserved in discrete siderite concretions (Fig. 6.1, 6.2). Siderite-replaced crinoids also may be weathered free, implying that the calcite plates were replaced by siderite even though the specimen was totally encased in shale (Fig. 2.1). Crinoids also may occur in a siderite bed completely or partially replaced by siderite (Fig. 3). If partially replaced, several variations of replacement occur. For example, the outer portion of crinoid plates may be replaced by siderite, the inner portion is calcite (Fig. 6.3, 6.4), or the opposite may occur (Fig. 7.2). Alternatively, the distribution of siderite and calcite may be more random (Figs. 6.4, 7.2). In beds with crinoids preserved in both calcite and siderite, the calcite is secondary. The original crinoid plates, which were composed of a single crystal of calcite, have been replaced by a fine- to medium-grained calcite (Figs. 6.4, 7.2). Further, in some instance, the surrounding siderite bed may have calcite-filled fractures that are connected to calcite portions of crinoid plates (Figs. 6.4, 7.2). A few crinoids in siderite concretions are also replaced with pyrite.

Figure 6. Siderite preservation of crinoids in the Wooster Shale (all specimens uncoated). (1) CMNH 18017, two parts of a siderite concretion with an unidentifiable camerate crinoid. (2) Cactocrinus woosterensis n. sp., OSU 53550, paratype, specimen in a siderite concretion (compare to Fig. 2.2). (3, 4) CMNH 18018 bed completely replaced by siderite with crinoids partially replaced (white is calcite, gray coloration at arrow is pyrite, remainder of bed is siderite); (3) upper part of bedding surface with partially replaced columnals; on left side of bed an upside down Cactocrinus woosterensis n. sp., specimen replaced with siderite, partially buried, and with “pyrite rot” destroying the calyx; (4) cross section through bed illustrating various modes of columnal replacement with siderite; note infilling of fractures with calcite. Scale bar represents 5.0 mm in (1, 2, 4) and 10.0 mm in (3).

Figure 7. CMNH 18019 bed largely replaced by siderite (white is calcite). (1) Upper part of bedding surface completely replaced; (2) cross section through bed illustrating various modes of replacement of columnals with siderite; note infilling of fractures with calcite; bed is slightly tilted to illustrate that the pattern of replacement on the cross section continues into the bed. Scale bar represents 5.0 mm in (2), 10.0 mm in (1).
Because most of the specimens with moldic preservation disintegrate with weathering on the outcrop, it is impossible to determine the most common mode of preservation in the Wooster Shale Member. However, we presume that calcite preservation is the dominant mode. Based on collected specimens, preservation associated with siderite beds and preservation in shale are co-dominant, and preservation in small, definable siderite concretions (Fig. 6.1, 6.2) is the rarest.
The preservation of crinoids within siderite concretions has been described previously in the upper Carboniferous Francis Creek shale of Illinois (Lane, Reference Lane1969) and the upper Carboniferous Copan crinoid Lagerstätte of Oklahoma by Thomka and Lewis (Reference Thomka and Lewis2013). The Wooster Shale Member concretions closely resemble “Type 1 large concretions lacking distinct nuclei” (Thomka and Lewis, Reference Thomka and Lewis2013), which are oblong in shape, with the longest axes measuring at least seven cm and often significantly larger (up to 25 cm). These concretions are parallel to bedding, which Seilacher (Reference Seilacher2001) attributed to burial compaction during formation. The fossils in the Francis Creek shale, Copan Lagerstätte, and Wooster Shale Member siderite are uncrushed, indicating that the concretions formed early within the top meter of the sediment column. Thomka and Lewis (Reference Thomka and Lewis2013) proposed that these large siderite concretions formed in a stable alkaline environment rich in ferrous iron and bicarbonate over a prolonged period, likely during a time of sediment starvation.
Mississippian crinoid faunas
The Mississippian is the “Age of Crinoids” (Kammer and Ausich, Reference Kammer and Ausich2006), and crinoids were important faunal elements in many depositional settings during the Mississippian. Cuyahoga Formation faunas (Hall, Reference Hall1863; Ausich and Roeser, Reference Ausich and Roeser2012; Kammer and Roeser, Reference Kammer and Roeser2012) are preserved in siliciclastic facies, which is atypical for many Tournaisian crinoid faunas (e.g., Ausich, Reference Ausich, Hess, Ausich, Brett and Simms1999a, Reference Ausich, Hess, Ausich, Brett and Simmsb, and references therein). Rather, the composition of the Cuyahoga crinoids and the paleoenvironmental setting are more similar to some early Viséan faunas in siliciclastic settings, such as at Crawfordsville, Indiana (van Sant and Lane, Reference van Sant and Lane1964; Lane, Reference Lane1973; Ausich, Reference Ausich, Hess, Ausich, Brett and Simms1999c).
In comparison to the Meadville Shale Member of the Cuyahoga Formation, the Wooster Shale Member crinoid fauna is small (30 in the former and nine in the latter) (Table 2). Representatives of every major clade occur in the Meadville Shale Member, and the Wooster Shale Member has every clade represented except the disparids. As discussed in detail above, three new species of camerates and two new species of eucladids (Cyathoformes incertae sedis: ‘Poteriocrinida’) are described from the Wooster Shale Member fauna. Only one taxon, Cyathocrinites simplex, is shared in both faunas.
Another interesting aspect of the Wooster Shale Member crinoid fauna is the distribution of new species among clades. Kammer et al. (Reference Kammer, Baumiller and Ausich1997, Reference Kammer, Baumiller and Ausich1998) identified differential species longevities among different major crinoid clades. Disparids and primitive cladids (cyathoformes incertae sedis: Cyathocrinida, herein) have longer species longevities and were regarded as niche generalists. In contrast, camerates, flexibles, and advanced cladids (Cyathoformes incertae sedis: Poteriocrinida, herein) had shorter species durations and were regarded as niche specialists. Sample size of the Wooster Shale Member fauna is too small to suggest any robust conclusions. However, all five new species (Agaricocrinus murphyi n. sp.; Cactocrinus woosterensis n. sp.; Cusacrinus brushi n. sp.; Decadocrinus laevis n. sp.; and Decadocrinus inordinatus n. sp.) that we described from the Wooster Shale Member belong to niche specialist clades with shorter species duration, which is consistent with the conclusions of Kammer et al. (Reference Kammer, Baumiller and Ausich1997, Reference Kammer, Baumiller and Ausich1998).
Acknowledgments
Our collection of Wooster Shale Member crinoids is a combination of material collected by the authors and from the collections of James L. Murphy and Gary Meszaros, who donated their specimens to the Orton Geological Museum and the Cleveland Museum of Natural History, respectively. We are grateful for the help that Nigel Brush provided to help us understand the geographic distribution of Wooster Shale Member. We thank T.W. Kammer and G.C. McIntosh for their help as we considered the taxonomic placement of some Wooster crinoids. We also thank T.W. Kammer, D.L. Meyer, and S. Zamora, whose reviews greatly improved this manuscript.
Declaration of competing interests
The authors declare none.
Data availability statement
Data available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.qnk98sfmr.












