Introduction
Arthritis has a significant global burden(Reference Cross, Smith and Hoy1–Reference Scotti, Franchi and Marchesoni3). It includes a wide variety of clinical conditions, including osteoarthritis (OA), rheumatoid arthritis (RA) and seronegative arthropathies (SA) (psoriatic, reactive, ankylosing spondylitis (AS) or inflammatory bowel disease (IBD)-related). The pathologies of these diseases are highly variable, but all include a component of joint inflammation. Patients with high disease activity typically have impaired quality of life and activities of daily living(Reference Clynes, Jameson and Edwards4–Reference Kaye, Callahan and Nance7).
The low- or anti-inflammatory diet (both names are synonymous) is based on principles of the Mediterranean diet. It emphasises on foods high in antioxidants, polyphenols, carotenoids, omega-3 fatty acids (long chain), foods low in the glycaemic index and promotes the utilisation of extra virgin olive oil as the main source of fat(Reference Radd-Vagenas, Kouris-Blazos and Singh8). Furthermore, the diet advises the reduction or minimisation of refined carbohydrates, fast foods, foods high in trans-fat and saturated fat, alcoholic beverages, sugary beverages and processed meats(Reference Musumeci, Trovato and Pichler9–Reference Buyken, Flood and Empson11). Due to the low-inflammatory effects, it is hypothesised that the diet may be beneficial in providing symptom-relief for patients with arthritis. Consequently, many patient-centred medical websites promote such a diet for patients with arthritis(12–Reference Krans and Kinman15).
Previous research suggests that obese patients with OA can successfully lose weight and report symptom improvement following a diet-based weight loss intervention(Reference Alrushud, Rushton and Kanavaki16–Reference Messier, Mihalko and Legault18). This is also the case for those who suffer from RA(Reference Sköldstam, Hagfors and Johansson19) and psoriatic arthritis(Reference Klingberg, Bilberg and Bjorkman20). Furthermore, research suggests that a low-inflammatory diet may alleviate arthritic symptoms(Reference Adam, Beringer and Kless21,Reference Dyer, Davison and Marcora22) . It is unclear; however, if symptom-relief is related to change in inflammation, change in weight, change in adipose tissue, or the quality or type of dietary intervention.
The overall objective of this review is to assess the health impact of a low-inflammatory diet intervention (full-diet or supplements) compared to usual diet or other dietary interventions. Specifically, the health impact includes the effect on inflammatory biomarkers, joint symptoms, quality of life and weight change in adults who suffer from OA, RA or seronegative arthropathy (psoriatic, reactive, AS or IBD-related) affecting any joint in the body, within the first 6 months since the commencement of the intervention.
Methods
The protocol was registered on PROSPERO prior to commencement of the article screening (Reg. No. CRD42019136567). The PRISMA guidelines were used to report this systematic review(Reference Shamseer, Moher and Clarke23).
Eligibility criteria
The eligibility criteria for included studies are summarised in Table 1. Studies that investigated the effects of a low-inflammatory diet intervention in adults with arthritis were assessed for changes in weight, joint symptoms and inflammatory biomarkers. Interventions included any low-inflammatory dietary intervention compared to diet as usual or other forms of dietary interventions. Prospective studies with their full-text articles published in English were eligible for inclusion.
Table 1. Systematic review study eligibility criteria

Information sources
The following electronic databases were searched from inception till 10 July 2019; MEDLINE, EMBASE, Cochrane Database of Systematic Reviews (potentially eligible reviews had their references reviewed for eligible studies), Cochrane Central Register of Controlled Trials (CENTRAL), CINAHL. Reference lists of included studies as well as the grey literature (Proceedings of the American Society of Nutrition and International Congress of Dietetics and a Google Scholar search) were also reviewed.
Search strategy
The search strategy, using a combination of key and text words, was formulated with the help of a medical librarian. The syntax of the search for each database is found in Supplementary Tables and Figures.
Selection process
Ascertainment of eligible studies was conducted independently by two reviewers (F.G. and M.K.). The titles and abstracts of articles were screened for eligibility. Once potential studies were identified, both reviewers obtained full-text articles to assess and discuss their eligibility. If there was a disagreement between the two reviewers, a resolution was sought by discussing the study with a third reviewer (J.M.N., S.A. or V.M.F.).
Data collection process and management
Data extraction from eligible studies and subsequent collation was completed by the two reviewers independently using standardised forms. Any discrepancies in the data were resolved through discussion. Attempts were made to contact the corresponding authors of studies where data were missing. When extracting data from trials, only intention-to-treat (ITT) data was utilised. However, if ITT data were not provided in the article or on request, then per-protocol data were utilised.
Data items
The following data items were extracted from included articles: author, reference, country, population characteristics (such as age, gender, diagnosis and joints affected), study design, trial size (at baseline and size for analysis for both intervention and control/comparator groups), intervention characteristics such as details of the intervention, intervention duration, quality of intervention (see Section ‘Risk of bias’), details of the control/comparator (e.g. compared to normal diet or other diet programs, size of group and duration of control/comparator), duration of follow-up, outcome details (refer to Section ‘Outcomes and prioritisation’), details needed for assessment for risk of bias and author contact details.
Outcomes and prioritisation
Continuous outcomes were presented as mean differences (MD), standardised mean differences (SMD), for data reported with different tools, along with standard deviations or 95 % confidence intervals (95 % CIs). When standard deviations were not reported, these were calculated from other available data, such as a 95 % CI, standard error, or a P value(Reference Higgins, Deeks, Higgins, Thomas and Chandler24–27). There were three primary outcomes:
1. Weight loss – converted to metric units (km) when necessary. Body mass index (BMI) was also extracted, using change scores whenever possible.
2. Blood inflammatory markers – the main markers of interest in this review were C-reactive protein (CRP, both high sensitivity CRP and standard CRP, measured in mg/ml) as well as Interleukin-6 (IL-6, measured in pmol/l) since these are the most widely reported in the literature(Reference Burghardt, Kazim and Rüther28). Data for other inflammatory markers were also extracted including (but not limited to) IL-1β and TNF-alpha.
3. Joint symptoms – using a validated joint pain/function score for arthritis populations, such as Oxford(Reference Dawson, Fitzpatrick and Carr29,Reference Dawson, Fitzpatrick and Murray30) , or WOMAC scores(Reference McConnell, Kolopack and Davis31).
Interventional trials had their outcome data extracted at baseline, during the dietary intervention, and at the end of dietary intervention (before or at 6 months). Outcomes reported with varying timepoints were grouped as 0–2, 2–4 and 4–6 months. Scoping of the literature indicated most dietary interventions were for 3 months(Reference Sköldstam, Hagfors and Johansson19,Reference Adam, Beringer and Kless21) ; however, interventions existed for 0–3(Reference Christensen, Astrup and Bliddal32) and 3–6 months(Reference García-Morales, Lozada-Mellado and Hinojosa-Azaola33–Reference Riecke, Christensen and Christensen35). In light of this, and the associated clinical significance of the timepoint, 2–4 months were chosen as the primary timepoint for all outcomes.
Risk of bias in individual studies
The randomised trials were assessed for bias using the Cochrane Handbook's RoB Version 2 checklist(Reference Higgins, Sterne and Savović36), while prospective studies were assessed using the Cochrane Handbook's ROBINS-I tool(Reference Sterne, Hernán and Reeves37). Each assessment was conducted independently by two reviewers. Any discrepancy was resolved over the discussion and an arbitrator was used if necessary.
As an indicator of therapeutic validity, the quality of the intervention utilised in the studies was also assessed. The areas of interest were informed by an earlier review(Reference Radd-Vagenas, Duffy and Naismith38) and included whether (i) a dietitian was involved in the design of the dietary intervention; (ii) the dietary intervention was a partial or full simulation of the low-inflammatory diet; (iii) there was the monitoring of adherence to the dietary intervention and (iv) a validated tool was utilised to measure dietary adherence.
Data synthesis
Outcomes were pooled from at least two studies in random-effects meta-analysis. The inverse variance method was utilised when conducting a meta-analysis of data from different studies. For the purposes of meta-analysis, timepoints for outcomes were stratified at 0–2, 2–4 and 4–6 months. Studies were pooled when there was satisfactory clinical homogeneity (similar study design, population and intervention); otherwise, they were discussed narratively. Statistical heterogeneity was assessed using the I 2 statistic.
Changes in inflammatory biomarkers and weight were derived by subtracting mean baseline values from subsequent values; thus, a negative change implied improvement in health. However, for joint symptoms, a decrease in score could be an improved outcome (such as WOMAC(Reference McConnell, Kolopack and Davis31)), while an increased score in another tool could also represent improvement (such as Oxford Knee Score(Reference Dawson, Fitzpatrick and Murray30)). Thus, tools that reported poor health in the opposite direction to the majority of other tools had their change scores multiplied by −1(Reference Higgins, Thomas, Chandler, Higgins, Li and Deeks39) (Supplementary Table S1).
Some data in the selected studies were not presented in a usable form without modification. For example, change in weight (as a percentage) was reported instead of pre- and post-weight. To ensure the same variables were extracted from all studies, these variables were converted to a usable form (e.g. the post-weight was calculated from the reported basal weight and percentage change in weight). When this could not be done or missing data was encountered, missing data were requested from corresponding authors. No imputation of missing data was undertaken. Data analysis was completed using RevMan 5.3 software(40).
A priori, we planned to explore heterogeneity by performing a subgroup analysis of (i) osteoarthritis v. inflammatory/seronegative arthritis, (ii) different duration of intervention (0–2 months v. 2–4 months (timepoint of interest) v. 4–6 months) to assess possible dose-response effects and (iii) RCTs v. other prospective studies. After studies were identified, we also planned subgroup analysis of (i) diets that resulted in weight loss v. diets without weight loss and (ii) full low-inflammatory diet v. partial diet (e.g. anti-inflammatory supplements).
Meta-biases and confidence in cumulative evidence
Assessment of publication bias was planned, but there were insufficient studies (<10 studies), to construct a funnel plot or perform Egger's regression test. The methodology of the Grading of Recommendations Assessment, Development and Evaluation (GRADE)(Reference Guyatt, Oxman and Vist41) working group was utilised in assessing the quality of the evidence presented in the review. The evidence was graded as either very low, low, moderate or high-quality evidence.
Results
Study selection
Of the 453 citations retrieved from multiple sources, seven articles(Reference Sköldstam, Hagfors and Johansson19,Reference Adam, Beringer and Kless21,Reference Dyer, Davison and Marcora22,Reference García-Morales, Lozada-Mellado and Hinojosa-Azaola33,Reference Du, Smith and Avalos42–Reference Schell, Scofield and Barrett44) met the eligibility criteria (Fig. 1). Five articles were randomised trials(Reference Sköldstam, Hagfors and Johansson19,Reference Dyer, Davison and Marcora22,Reference García-Morales, Lozada-Mellado and Hinojosa-Azaola33,Reference Du, Smith and Avalos42,Reference Schell, Scofield and Barrett44) , two were pre–post trials(Reference Adam, Beringer and Kless21,Reference McKellar, Morrison and McEntegart43) . These trials had a total of 468 patients (259 in the intervention and 226 in the control). Requests for additional information were made to four authors; one responded.

Fig. 1. PRISMA flow diagram of the article screening and selection process for the systematic review.
Study characteristics
The age range for the participants was from 30 to 90 years. Five of the seven trials included both sexes; two studies included women only. ‘Women only’ studies focused on RA, while the other trials focused either on RA or OA. Intervention duration ranged from 3 to 6 months. Five of the seven studies had a low-inflammatory diet (including ‘anti-inflammatory’ and ‘Mediterranean’ diet) as the intervention, while two studies utilised low-inflammatory supplements (i.e. strawberry and blueberry powders). All intervention diets were non-calorie restricted. Control/comparator diets included the usual diet or placebo powders. There were a total of 259 and 226 participants in the intervention and control/comparator analyses, respectively. The characteristics of the included trials are found in Table 2. One of the studies(Reference Schell, Scofield and Barrett44) was a cross-over trial. As per Cochrane guidelines, the data were extracted as if the study was a parallel-group trial of intervention v. control(Reference Higgins, Deeks, Altman, Higgins, Thomas and Chandler45).
Table 2. Study characteristics of included studies of the systematic review

RCT, Randomised Control Trial; M, Male; F, Female; LID, Low-inflammatory Diet; AID, Anti-inflammatory Diet; MD, Mediterranean Diet; BMI, Body Mass Index.
Risk of bias
From the randomised trials, one study(Reference Schell, Scofield and Barrett44) had an overall low risk of bias, while the remaining studies(Reference Sköldstam, Hagfors and Johansson19,Reference Dyer, Davison and Marcora22,Reference García-Morales, Lozada-Mellado and Hinojosa-Azaola33,Reference Du, Smith and Avalos42) had an overall high risk of bias (Supplementary Fig. S1). For the prospective non-randomised trials, one(Reference Adam, Beringer and Kless21) had an overall moderate risk of bias, while the other(Reference McKellar, Morrison and McEntegart43) had an overall serious risk of bias (Supplementary Table S2).
In assessing the quality of the dietary interventions specifically, only one study(Reference García-Morales, Lozada-Mellado and Hinojosa-Azaola33) clearly stated the utilisation of a dietitian in designing the intervention. Two studies(Reference Dyer, Davison and Marcora22,Reference García-Morales, Lozada-Mellado and Hinojosa-Azaola33) were a full simulation of a low-inflammatory diet, while other studies were only partial. While all the studies that assessed participant dietary compliance did so in various ways, only one study(Reference McKellar, Morrison and McEntegart43) utilised a validated tool (Supplementary Table S3).
Results of individual studies
Individual study level data is provided in Supplementary Tables S4 and S5. Synthesised results are reported below.
Synthesis of results
Table 3 presents the GRADE Summary of Findings for each outcome. SMD values were converted back to familiar clinical measurements(Reference Higgins, Deeks, Higgins, Thomas and Chandler24,Reference Schünemann, Vist, Higgins, Higgins, Thomas and Chandler25) . Overall, the outcome results were of very low quality as assessed by the GRADE system. There were a small number of studies included in the analysis, and high heterogeneity was evident for most comparisons.
Table 3. GRADE Summary of Findings
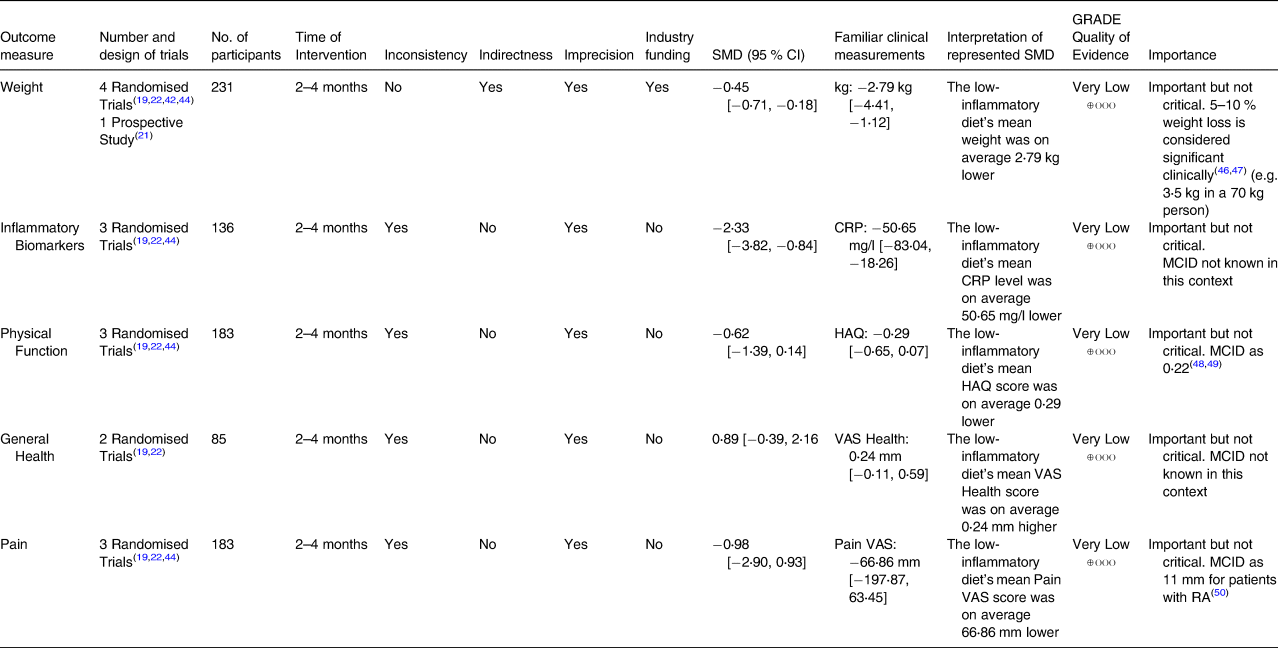
Outcomes utilised were data from 2 to 4 months of intervention. The ‘GRADE Quality of Evidence’ was derived based on the GRADE methodology. (Interpretation of SMD results were calculated by multiplying the SMD with sd of the tool its being re-expressed as. sd was derived from the pooled mean and 95 % CI of all study groups (intervention and control groups) for that specific tool.)
Weight
A standardised analysis combining weight (kg) and BMI across the studies after 2–4 months of the intervention, showed very low-quality evidence favouring weight change in the low-inflammatory group, with a statistically significant SMD of −0⋅45 ([95 % CI −0⋅71, −0⋅18], P = 0⋅0009) compared to the usual diet group (Fig. 2).
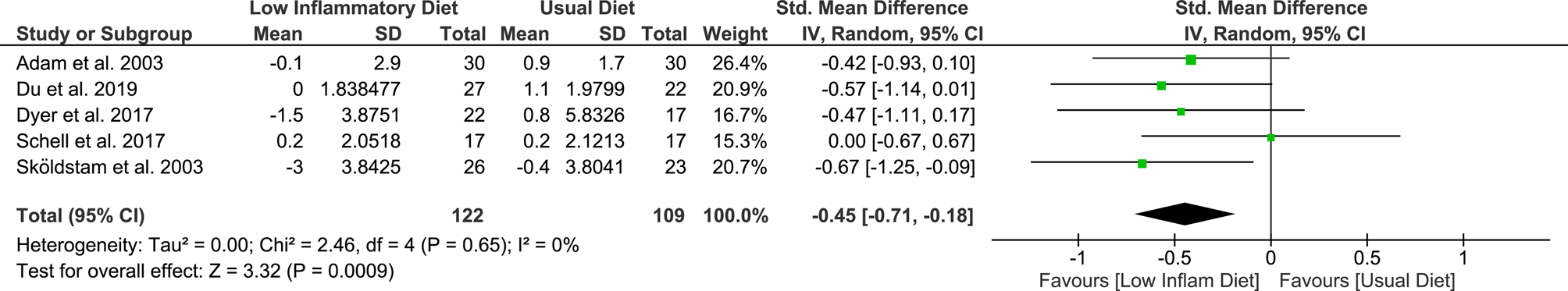
Fig. 2. Meta-analysis of weight change when comparing low-inflammatory diet to usual diet following 2–4 months of intervention/control.
A subgroup analysis assessing weight change according to diagnosis indicated patients diagnosed with RA and OA had a weight change following a low-inflammatory diet (RA – SMD −0⋅53 [95 % CI −0⋅91, −0⋅14], P = 0⋅007; OA – SMD −0⋅37 [95 % CI −0⋅73, −0⋅01], P = 0⋅04; Supplementary Fig. S2), although there were no differences between subgroups (test for subgroup difference, P = 0⋅57). When analysing based on study type, only randomised trials favoured the low-inflammatory diet (RCTs – SMD −0⋅46 [95 % CI −0⋅76, −0⋅15], P = 0⋅004; prospective trial – SMD −0⋅42 [95 % CI −0⋅93, 0⋅10], P = 0⋅11; Supplementary Fig. S3). Further subgroup analysis based on the nature of intervention (full v. partial low-inflammatory diet) can be found in Supplementary Fig. S9. Weight change at 2–4 months favoured full simulation of low-inflammatory diet, but not partial simulation (full simulation SMD −0⋅51 [95 % CI −0⋅84, −0⋅18], P = 0⋅002; partial simulation SMD –0⋅31 [95 % CI −0⋅87, 0⋅24], P = 0⋅27; Supplementary Fig. S9).
Only one study(Reference García-Morales, Lozada-Mellado and Hinojosa-Azaola33) reported data for weight change after 4–6 months of intervention. The calculated change scores can be found in Supplementary Tables S4 and S5.
Inflammatory biomarkers
There was very low-quality evidence that reduction in inflammatory biomarkers at 2–4 months favoured the low-inflammatory diet; SMD −2⋅33 ([95 % CI −3⋅82, −0⋅84], P = 0⋅002; Fig. 3). An individual meta-analysis for each type of inflammatory biomarker did not favour either diet. At 2–4 months, the low-inflammatory group had an MD in change scores of −2⋅46 mg/l ([95 % CI −7⋅15, 2⋅24], P = 0⋅31), −3⋅41 pg/ml ([95 % CI −7⋅09, 0⋅28], P = 0⋅07) and –4⋅56 pg/ml ([95 % CI −12⋅43, 3⋅32], P = 0⋅26) in CRP, IL-6 and IL-1β, respectively (Supplementary Fig. S4).

Fig. 3. Meta-analysis of inflammatory biomarker change when comparing low-inflammatory diet to usual diet following 2–4 months of the intervention/control. In this analysis of change scores, Skoldstam's CRP results, Schell's IL-6 and Dyer's IL-6 results were utilised.
In a subgroup analysis based on diagnosis, both the OA group (SMD −3⋅09 [95 % CI −5⋅92, −0⋅26], P = 0⋅03) and the RA group (SMD −1⋅10 [95 % CI −1⋅71, −0⋅49], P = 0⋅0004; Supplementary Fig. S5) had reductions in inflammatory biomarkers (independent of weight change); the differences between the group were not significant (P = 0⋅18; Supplementary Fig. S5). Additional subgroup analyses (based on the nature of intervention and weight loss during intervention) can be found in Supplementary Fig. S10. Inflammatory biomarkers at 2–4 months favoured both partial and full simulation of low-inflammatory diet (partial simulation SMD −4⋅60 [95 % CI −5⋅94, −3⋅26], P < 0⋅00001; full simulation SMD −1⋅40 [95 % CI −2⋅00, −0⋅80], P < 0⋅00001; Supplementary Fig. S10). Change in biomarkers was favoured in both the presence and absence of weight loss in the low-inflammatory diet (Supplementary Fig. S10).
Only one study(Reference Sköldstam, Hagfors and Johansson19) reported inflammatory marker change scores after 0–2 months for intervention. The calculated change scores can be found in Supplementary Tables S4 and S5.
Patient-reported outcome measures
At 2–4 months, very low-quality evidence suggests that physical outcome measures did not favour either diet (SMD −0⋅62 [95 % CI −1⋅39, 0⋅14]), P = 0⋅11; Fig. 4). This analysis was completed utilising the intervention and control change scores of Arthritis Impact Measurement Scales (AIMS-2) physical score from Dyer et al. (2017; intervention mean −0⋅1, standard deviation (sd) 0⋅2931; control mean −0⋅1, sd 0⋅3839(Reference Dyer, Davison and Marcora22)) and Health Assessment Questionnaire (HAQ) scores from Schell et al. (2017; intervention mean −0⋅2, sd 0⋅1414; control mean 0, sd 0⋅1414(Reference Schell, Scofield and Barrett44)) and Skoldstam et al. (2003; intervention mean −0⋅1, sd 0⋅1266; control mean 0, sd 0⋅1769(Reference Sköldstam, Hagfors and Johansson19); Supplementary Tables S4 and S5). Subgroup analysis by diagnosis demonstrates inconclusive results for OA (SMD −0⋅65 [95 % CI −2⋅00, 0⋅70], P = 0⋅34). However, in RA, physical outcome measures favoured the low-inflammatory diet (SMD −0⋅65 [95 % CI −1⋅22, −0⋅07], P = 0⋅03; Supplement Fig. S6). The test for subgroup differences produced a P value of 0⋅99, suggesting that the results did not favour either the OA or RA group (Supplementary Fig. S6). Additional subgroup analysis based on the nature of intervention (full v. partial low-inflammatory diet) and the presence/absence of weight loss in the intervention can be found in Supplementary Fig. S11. Physical function at 2–4 months favoured the partial simulation of low-inflammatory diet and not the full simulation (partial simulation SMD −1⋅38 [95 % CI −2⋅14, −0⋅62], P = 0⋅0004; full simulation SMD −0⋅29 [95 % CI −0⋅92, 0⋅34], P = 0⋅37). Furthermore, change in physical function was favoured in the absence of weight loss in the low-inflammatory diet and not in the presence of weight loss in the low-inflammatory diet.

Fig. 4. Meta-analysis of physical function change when comparing low-inflammatory diet to usual diet following 2–4 months of the intervention/control.
A meta-analysis was also completed assessing change in pain scores. At 2–4 months, there was very low-quality evidence that change in any pain score favoured neither diet (SMD −0⋅98 [95 % CI −2⋅90, 0⋅93], P = 0⋅31; Fig. 5). In a subgroup analysis based on diagnosis, pooled pain scores were different; the RA group had a statistically significant SMD of −2⋅81 ([95 % CI −3⋅60, −2⋅02], P < 0⋅00001; Supplementary Fig. S8) favouring the low-inflammatory diet. Subgroup difference analysis resulted in a P value = 0⋅0004, suggesting that the results favoured the RA group over the OA group. Furthermore, subgroup analysis based on the nature of intervention (full v. partial low-inflammatory diet) and the presence/absence of weight loss in the intervention can be found in Supplementary Fig. S12. Pain scores at 2–4 months favoured only the partial simulation and not the full simulation of low-inflammatory diet (partial simulation SMD −0⋅77 [95 % CI −1⋅47, −0⋅07], P = 0⋅03; full simulation SMD −1⋅11 [95 % CI −4⋅41, 2⋅19], P = 0⋅51). Additionally, change in pain scores was favoured in both the presence and absence of weight loss in the low-inflammatory diet.

Fig. 5. Meta-analysis of pain score change when comparing low-inflammatory diet to usual diet following 2–4 months of the intervention/control.
At 2–4 months, there was very low-quality evidence that general health outcomes favoured neither the diet group (SMD 0⋅89 [95 % CI −0⋅39, 2⋅16]), P = 0⋅17; Supplementary Fig. S7). Results for 0–2 months of intervention were calculated for physical function and pain scores (Supplementary Figs. S13 and S14); physical function and pain scores favoured neither the diet group. One study(Reference García-Morales, Lozada-Mellado and Hinojosa-Azaola33) reported change scores in weight change, physical outcome, pain and general health at 4–6 months of intervention. It showed that the health benefits for the low-inflammatory diet may be present at 4–6 months of intervention; however, a meta-analysis to assess this could not be conducted due to insufficient studies. Calculated change scores can be found in Supplementary Tables S4 and S5.
Discussion
This systematic review provides a comprehensive assessment of the dearth of literature concerning the health effects of a low-inflammatory diet in people with arthritis. In comparison to a usual diet, very low-quality GRADE evidence suggests that a low-inflammatory diet is associated with more weight loss, lower inflammation, improved physical function measures (RA only) and reduced joint pain (RA only). We also assessed the effect sizes at different periods and found that changes in outcomes were found as early as 2–4 months following commencement of the intervention.
Obesity is common among those who suffer from OA(51) and RA(Reference de Resende Guimarães, Rodrigues and Gomes52,Reference Stavropoulos-Kalinoglou, Metsios and Koutedakis53) . In the meta-analysis, one study(Reference Schell, Scofield and Barrett44) specifically recruited obese patients with knee OA, while another study's baseline characteristics indicate the inclusion of obese participants(Reference Du, Smith and Avalos42). Previous research indicates calorie-restricted diets are effective in producing weight loss in OA patients(Reference Messier, Mihalko and Legault18,Reference Messier, Loeser and Miller54) . A recent network meta-analysis suggests that low-inflammatory diets, though often not targeting weight loss, may also usefully reduce weight within 6 months of commencement though the effects diminish by 12 months(Reference Thijssen, van Caam and van der Kraan55). We also found in this meta-analysis that the low-inflammatory diet could procure weight loss. This is important because such a diet can be useful for not only reducing the inflammatory load induced by diet but also the inflammatory load associated with excess adipose tissue(Reference Thijssen, van Caam and van der Kraan55).
Inflammatory biomarkers are elevated in the serum of patients with arthritis. IL-6 and CRP have roles in the pathogenesis of arthritis and are associated with disease activity(Reference Yoshida and Tanaka56,Reference Daghestani and Kraus57) . Reduction in inflammatory biomarkers may indicate reduced disease severity and progression. Previous research indicates that a calorie-restricted diet and exercise reduces IL-6 levels in obese OA patients(Reference Messier, Mihalko and Legault18). One clinical trial highlighted the reduction of IL-6 and hsCRP following significant weight loss secondary to gastric surgery(Reference Richette, Poitou and Garnero58). While improvements in inflammatory biomarkers may be confounded by the concomitant weight loss, our results reveal improvements in biomarkers can occur independent of weight change.
For RA patients, improvement in pain and physical function measures were greater with those following a low-inflammatory diet. Statistically significant benefits were not seen in people with OA. Our findings are not consistent with studies where people with OA were given low or calorie-restricted diets and significant improvements in pain and function were observed(Reference Messier, Mihalko and Legault18,Reference Messier, Loeser and Miller54,Reference Gandler, Simmance and Keenan59) . These contradictory findings may suggest that for OA patients to obtain physical function and pain benefits from a dietary intervention, calorie restriction needs to be incorporated. Interestingly, general health measures were not associated with either the diet group; however, only two papers were part of the meta-analysis.
Varied duration of the interventions was used to assess the potential dose-response effect. As stated in the results, change scores were extracted for three time periods; however, the majority of the data were applied to the 2–4 months period. Comparing the meta-analysis results from 0–2 months and 2–4 months, the data suggest that change in outcomes were evident after 2 months of intervention. Due to lack of data beyond 4 months, we could not determine what the ideal duration of intervention is to produce the most significant improvements in outcomes.
Most of the dietary interventions only provided a partial simulation of the low-inflammatory diet and did not involve a dietitian in its design; hence, the therapeutic validity of these dietary interventions is questionable. Treatment fidelity is also questionable as there was a lack of a validated tool used to assess dietary compliance during the trials. Strengths of this review are that it covers a topic of great public interest given the interest in diet and arthritis generally(12,13) and includes a pre-registered protocol which was performed and reported within current guidelines (including PRISMA guidelines and Cochrane Handbook). Furthermore, change scores were imputed from the varied duration of intervention/control (to assess potential dose-effect responses), and we dealt with heterogeneity where possible using sensitivity analyses. Furthermore, strengths include the a priori design which was pre-registered, the comprehensive assessment of the quality of the evidence and incorporation of GRADE, and the standardisation of outcomes in order to include them in pooled effect sizes – which were then re-converted into meaningful clinical measures. Limitations of the review are that the included studies had small samples and were predominantly of poor quality, which resulted in imprecision given the small number of participants (less than 400), a heterogeneous analysis and missing data – all which limit the confidence in our overall conclusions.
Conclusion
In this meta-analysis of randomised and prospective trials, there is the very low quality of evidence, suggesting that a low-inflammatory diet is associated with greater weight loss, a greater decrease in inflammatory biomarkers, greater improvement in joint pain measures (RA only) and greater improvement in physical function measures (RA only) compared to usual diets. Health benefits appear to favour those with rheumatoid arthritis more than osteoarthritis. Although many patient-oriented information sources promote the low-inflammatory diet for arthritis patients, this review highlights the poor quality of evidence behind these public health recommendations. Furthermore, high-quality trials are needed evaluating a dietitian-led, low-inflammatory diet on a combination of laboratory and patient-reported outcomes, particularly in osteoarthritis.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/jns.2020.31.
Acknowledgements
The authors acknowledge the assistance provided by the librarian at Liverpool Hospital Library for their guidance in preparing the search syntax for the databases. The authors involved in the review are affiliated with the following institutions:
– Whitlam Orthopaedic Research Centre, Liverpool Hospital, Liverpool, NSW, Australia.
– South West Sydney Clinical School, UNSW, Australia.
– St George and Sutherland Clinical School, UNSW, Australia.
– Sydney School of Health Sciences, Faculty of Medicine and Health, University of Sydney, Australia.
– Ingham Institute of Applied Medical Research, Liverpool, NSW, Australia.
The author F.G. receives a funding from UNSW University Postgraduate Award Scholarship. The author N.P. receives funding from Medibank Better Health Fund & ANZMUSC PhD Scholarship. The funders have no role in influencing the design or reporting of the systematic review.
The authors declared that they have no conflicts of interest.
The authors F.G., J.N., S.A., M.K. and V.M.F. were involved in the protocol development for the review. V.M.F. provided expertise regarding a low-inflammatory diet. J.N. and S.A. provided expertise in arthritis. F.G. and M.K. were involved in the article screening, selection and data extraction. F.G. was involved in data analysis. F.G. and N.P. were involved in the formulation of GRADE assessment. The authors F.G., J.N. and S.A. are involved in monitoring the review progress. All authors contributed to the writing of the related manuscript. J.N. and S.A. are the guarantors of the review.
Data relevant to this systematic review is found within the article and supplement file. Further data will be made upon request from the corresponding author.










