Colon cancer (CC), also known as colorectal cancer (CRC), is the third most commonly diagnosed malignancy and the fourth leading cause of cancer-related deaths worldwide(Reference Slattery, Yakumo and Hoffman1). The prevalence of CC among Iranian people was between 7 and 8 per 100 000 people, with a significant increase over the last several years(Reference Kolahdoozan, Sadjadi and Radmard2).
In most cases, CC occurs in people aged 50 years or older and the risk of CC recurrence is increased with age(Reference Becker3). It has been reported that about 6 to 7 % of CC cases have a genetic origin. Approximately 10 to 15 % of CRC occur in patients where at least one of his/her relatives also had CC(Reference Schwartz4). Also, some hereditary syndromes are also effective on the risk of CC including Lynch syndrome and familial adenomatous polyposis syndrome(Reference Borji5). In addition, some environmental factors such as alcohol consumption, smoking, physical inactivity, high-fat diet and consumption of red and processed meat are also considered as risk factors for CRC(Reference Becker3). Recent studies reported that change in the expression level of some genes is also a mechanism involved in the effects of these environmental factors(Reference Doaei, Kalantari and Mohammadi6–Reference Akbari, Doaei and Gholamalizadeh8). Moreover, some people are at higher risk for CC because of their genotype(Reference Kalantari, Mohammadi and Rafieifar9). In other words, the development of CRC is a complex process that involves positive and negative interactions between genes and environmental factors. In the present study, the effects of the interactions between gene polymorphisms and red and processed meat consumption on the risk of CC have been reviewed.
Red and processed meat and colon cancer
Many studies have shown that there is a significant association between a red and processed meat-rich diet and CRC(Reference Le Marchand, Donlon and Seifried10, Reference Brink, Weijenberg and De Goeij11). This association has been attributed to several dietary factors, including heterocyclic amines, aromatic hydrocarbons produced during high temperature heating processes, N-nitrosamines that are found in many food products after nitrite addition and processed meat that contains high levels of preservatives. The polymorphisms in some genes involved in the metabolism of these components and risk of CC are discussed below.
N-acetyltransferases
Many studies have examined the enzymes involved in the metabolism of amines and heterocyclic amines and suggested a significant relationship between polymorphisms of these enzymes and risk of CC(Reference Wang, Iwasaki and Haiman12, Reference Ananthakrishnan, Du and Berndt13). Heterocyclic amines are produced during cooking meat at high temperatures. N-acetyltransferases (NAT) are important enzymes in the metabolic activation of heterocyclic amines, which are found in two forms of NAT1 and NAT2. The rs1495741 polymorphism of NAT2 was strongly related to its activity and the GG, AG and AA genotypes are classified as enzymes with rapid, intermediate and slow activity, respectively. In people with the GG genotype of this polymorphism, there is a strong association between the consumption of red meat and the risk of CRC(Reference Wang, Iwasaki and Haiman12–Reference Sørensen, Autrup and Olsen14). Another study reported that cooking meat at a high temperature increased the risk of CC in people with NAT2 gene polymorphisms(Reference Lilla, Verla-Tebit and Risch15). However, Barrett et al.(Reference Barrett, Smith and Waxman16) provide no support for the hypothesis that those with the fast phenotype of NAT2 are at increased risk of CRC.
A study was conducted on 147 CRC patients (seventy-six women and ninety men); the cancer risk in women was found to be lower in the NAT intermediate activity phenotype, but this difference was not found in men. It has also been reported that in people with the GG genotype of NAT2 G857A, meat intake more than three times per week increased CRC risk(Reference Procopciuc, Osian and Iancu17). However, some other studies failed to find any interaction between GG genotype, meat intake and CRC(Reference Da Silva, Felipe and de Lima18–Reference Chan, Tranah and Giovannucci20). For example, Chan et al.(Reference Chan, Tranah and Giovannucci20) reported that there was no interaction between the amount of meat consumed with NAT1 and NAT2 and the risk of developing CRC. Overall, it can be concluded that NAT2 gene polymorphisms may have a role in CRC risk, especially in people with high meat intake.
Cyclo-oxygenases
Cyclo-oxygenases (COX) play a key role in converting arachidonic acid into prostaglandins. Red meat contains a substantial amount of arachidonic acid and most probably is involved in the inflammatory response and initiation of CC especially in people with a polymorphism in the COX-1 and COX-2 genes. This polymorphism occurs in the promoter region of the gene, resulting in a possible increase in gene expression with consequent elevation of levels of the COX-2 protein. Individuals who carry the polymorphisms that could affect the expressions of COX-2 are more susceptible to CC(Reference Shalaby, Nounou and Ms21). There are two isoforms of the COX enzyme, COX-1 (or prostaglandin-endoperoxide synthase 1; PTGS1), that produces PG1, and COX-2 (or PTGS2), which produces PG2. The rs20417 (−765G > C) and rs5275 (8473T > C) polymorphisms of COX-2 play an important role in many cancers such as gastric cancer, prostate cancer and CRC. Some studies have also shown that the COX-2 rs1195AA genotype can also play a supportive role in the development of CRC. Makar et al.(Reference Makar, Poole and Resler22) showed that polymorphism rs20417 (−765G > C) in the COX-2 gene increases the risk of rectal cancer by up to two times higher than others. No significant relationship was reported between COX-1 gene polymorphisms and CRC in this study. In one meta-analysis study, there was a significant relationship between the COX-2 rs20417 polymorphism and the risk of CRC in an Asian population(Reference Peng, Yang and Lao23). Andersen et al.(Reference Andersen, Holst and Kopp24) suggested that the relationship between the COX-2 rs20417 polymorphism and the risk of CRC is influenced by dietary meat intake and COX-2 rs20417 risk allele carriers were at 8 % increased risk of CRC per 25 g/d higher red meat or processed meat intake. Generally, it can be concluded that COX-2 gene polymorphisms may have a role in CRC risk, especially in people with higher meat intake.
Cytochrome P450 2E1 and cytochrome P450 1A2
CRC is associated with environmental factors such as cigarette smoking, and consuming cooked meats and fish at high temperature. These factors result in the formation of carcinogenic compounds including polycyclic aromatic hydrocarbons, arylamines and heterocyclic amines. The cytochrome P450 (CYP) enzymes are critically important for the metabolism of these carcinogens by N oxidation(Reference Yoshida, Osawa and Kasahara25). CYP2E1 is an enzyme that plays a key role in the metabolism of nitrosamines and other carcinogens(Reference Hayashi, Watanabe and Kawajiri26). The RsaI polymorphism of CYP2E1 (C2 allele) is associated with an increased risk of CRC(Reference Hayashi, Watanabe and Kawajiri26, Reference Shahriary, Galehdari and Jalali27). The RsaI polymorphism has been shown to affect its transcription level. The variant type of this polymorphic site can enhance transcription and increase the level of CYP2E1 enzymic activity in vitro (Reference Le Marchand, Donlon and Seifried10).
Some studies have also shown that individuals carrying a variant of the C2 allele have lower enzyme activity. In the Hawaiian population, it has been shown that the risk of CC has decreased in subjects carrying the RsaI C2 allele(Reference Shahriary, Galehdari and Jalali27). On the other hand, a study in China showed that homozygous individuals for the C2 allele were also more likely to develop CRC(Reference Van der Logt, Bergevoet and Roelofs28). Moreover, in some other studies, no relationship was observed between RsaI polymorphisms and the risk of CRC(Reference Gao, Takezaki and Wu29). Interestingly, Morita et al.(Reference Morita, Le Marchand and Kono30) showed that there is a significant relationship between red meat consumption and an increased risk of CC in the individuals carrying the RsaI C2 allele. However, another study reported that no significant relationship was observed between the the CYP2E1 RsaI polymorphism, red meat consumption and CC(Reference Silva, Felipe and Pimenta31).
CYP1A2, a member of the cytochrome P450 mixed-function oxidase system, is involved in the metabolism of xenobiotics in the body(Reference Wang, Joshi and Corral32). Some studies have shown that individuals carrying CYP1A2 polymorphisms are at higher risk of developing rectal cancer but not for CC(Reference Le Marchand, Wilkinson and Wilkens33–Reference Cotterchio, Boucher and Manno35). In a case–control study on CYP1A2 polymorphisms, it was found that there was a significant relationship between the consumption of cooked meat at high temperature in −154A > C polymorphism carriers of CYP1A2 and the risk of CRC. Overall, it is possible that CYP2E1 and CYP1A2 gene polymorphisms may have a role in CRC risk, especially in people with higher meat intake.
Nucleotide excision repair pathway
The nucleotide excision repair (NER) pathway plays an important role in repairing damaged DNA. The NER pathway is a particularly important excision mechanism that removes DNA damage induced by UV light and environmental carcinogens(Reference Qiao, Spitz and Shen36). Xeroderma pigmentosum (XP) complementation group A (XPA), XP complementation group C (XPC) and XP complementation group D (XPD) are important enzymes in the NER pathway. There is a significant relationship between polymorphisms in XPA, XPC and XPD and a lower capacity of DNA repair. Numerous polymorphisms of NER genes have been identified and these changes individually or in combination may adversely affect NER fidelity, which could contribute to the risk of CRC. Four polymorphisms of these genes including A23G in XPA, Lys939Gln in XPC, and Lys751Gln and Asp312Asn in XPD have been identified that may have a significant relationship with the risk of CC(Reference Khan, Metter and Tarone37). For example, in a study conducted by Hansen et al.(Reference Hansen, Sørensen and Tjønneland38), a lower risk of cancer was reported in women with the Lys751Gln polymorphism of XPD. In homozygous individuals with the XPC Lys939Gln polymorphism, the risk of CC was increased by 3·7 times per 100 g/d increased intake of red meat. In the individuals carrying the wild-type allele, meat has no effect on CRC. No significant relationship was observed between other polymorphisms and CC.
Moreover, it was shown that people with a high consumption of red meat and XPD 312Asp and XPD 751Lys risk alleles have a higher chance of developing CRC than those with XPD 312Asn and XPD 751Gln alleles(Reference Joshi, Corral and Siegmund39). There is also a statistically significant interaction between Lys939Gln of XPC and A23G of XPA with red meat and processed meat intake and the risk of CC(Reference Hansen, Sørensen and Tjønneland38, Reference Steck, Butler and Keku40). Overall, it can be concluding that higher meat intake may have a role in CRC risk, especially in people with polymorphisms in genes involved in the NER pathway.
DNA mismatch repair (mutator S)
A DNA mismatch repair protein, also known as mutator S (MutS), participates in the DNA mismatch repair system. In a study conducted on the polymorphisms of this gene, it was found that some gene polymorphisms were associated with an increased risk of CC. Processed meat intake could increase CC risk in people with the MutS polymorphism(Reference Berndt, Platz and Fallin41). In another study, a significant relationship was observed between processed meat intake, the – polymorphism of the MutS gene and the risk of CC(Reference Laporte, Leguisamo and Kalil42). In general, it can be concluded that MutS polymorphisms may have a role in CRC risk, especially in people with higher processed meat intake.
Discussion
The presence of SNP associated with the metabolism and function of proteins could play an important role in the effects of red meat consumption on the risk of CC.
Several individual SNP have been associated with CC risk. It is plausible that a set of SNP derived from genetic pathways that are critical in colon carcinogenesis could contribute to the cancer risk. We investigated the role of polymorphisms involved in five metabolic pathways that are relevant for the activation or detoxification of carcinogens formed during red meat processing. The polymorphisms investigated in the present study were mostly functional polymorphisms that alter the expression of genes participating in metabolic pathways associated with carcinogenesis(Reference Goodman, Mechanic and Luke43).
Recent studies demonstrated the modifier role of NAT2 G857A, COX-2 rs20417, CYP2E1 RsaI, CYP1A2 154A>C, XPC Lys939Gln, XPA A23G and MutS T1036A on the effect of red meat consumption on CRC risk. However, some studies failed to identify an association between red meat consumption and the effect of these polymorphisms on CRC risk. Possible explanations for the discrepancy might include differences in meat variable definitions, and lack of stratification by tumour subsite in these studies. Moreover, other factors including frequency of turning the meat over during the cooking process, meat thickness, cut of meat, use of marinade or thawing meat in the microwave were not considered and may have contributed to these contradictory results(Reference Ho, Peacock and Massey44).
Conclusion
In conclusion, some gene polymorphisms may have a significant role in CRC risk, especially in people with higher processed meat intake. Increasing the knowledge on nutritional genomics can lead to the finding of new methods to prevent, treat and control of CC. A summary of descriptions of studies is presented in Table 1.
Table 1. Summary of study descriptions and outcomes
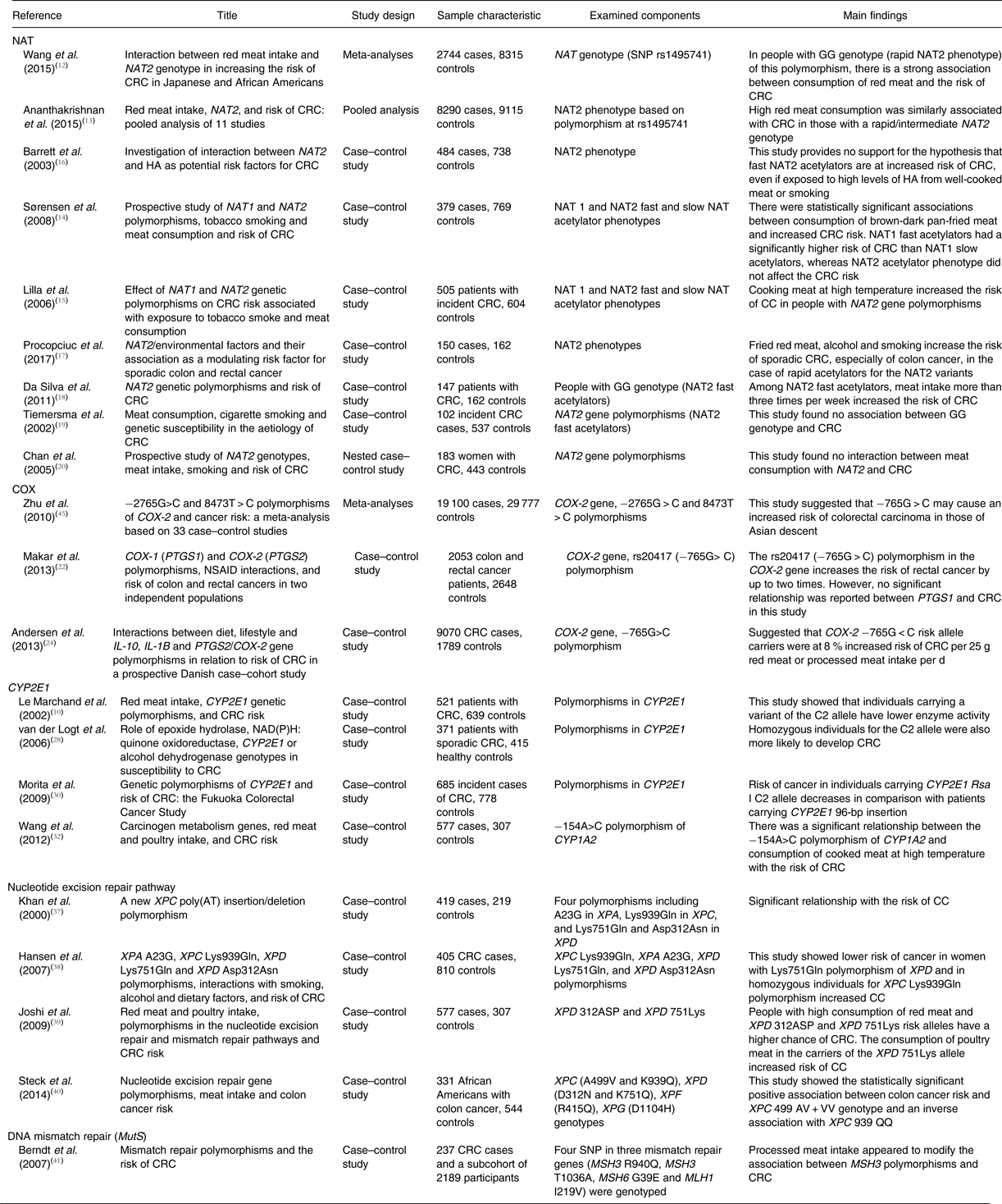
NAT, N-acetyltransferase; CRC, colorectal cancer; HA, heterocyclic amines; COX, cyclo-oxygenase; PTGS, prostaglandin-endoperoxide synthase; NSAID, non-steroidal anti-inflammatory drugs; CYP2E1, cytochrome P450 2E1; XP, xeroderma pigmentosum; MutS, mutator S; MSH, MutS homolog 3; MLH, MutL homolog 1.
Acknowledgements
This study was funded by the Student Research Committee of Shahid Beheshti University of Medical Sciences, Tehran, Iran (code 1396/54017). The authors contributed equally to this work.
None of the authors reported a conflict of interest related to the study.



