Introduction
Having a child is arguably a situation that bears a multifaceted issue with its psychological, economic and societal dimensions in a fair number of cultures, and it is deemed crucial assuming that it provides individuals with privilege and prestige in their society. Parents tend to teach their children about gender roles and the accepted social norms in their society from the moment they are born. Individuals who grew up with these cultural norms perceive infertility as the inability to fulfil the requirements of their masculinity for men, and the inability to meet the expectation of motherhood in society for women(Reference Karaca and Unsal1). From a scientific overview, infertility, one of the most common reproductive health disorders, is portrayed by the World Health Organization (WHO) as well as the International Committee for Monitoring Assisted Reproductive Technologies (ICMART) as the lack of clinical pregnancy proceeding 12 months or more of regular and unprotected sexual intercourse. Worldwide, the prevalence that is pertinent to infertility in partners who are at reproductive ages is between 8 and 12 %(Reference Vander Borght and Wyns2). The rates are 6⋅9–9⋅3 % in the countries classified as developing, and 3⋅5–16⋅7 % in the ones counted as developed(Reference Hosseini and Eslamian3). It has been reported that approximately one in eight couples seeks medical treatment after failing to conceive for a year(Reference Omar, Pal and Kelly4). When it comes to addressing the matter through the available methods, it would be fair to say that assisted reproductive techniques (ARTs) are most commonly used in the medical treatment of infertility. The technique in which infertile couples have the highest rate of having a baby with ART is in vitro fertilisation (IVF)(Reference Chen and Heilbronn5). Howbeit, surgery, drug therapy and treatment with IVF or other ART for infertility do not yield pregnancy and/or live birth all the time(Reference Ried6). Despite the perception that the cause of infertility mainly belongs to the female partner, it is indeed a fact that it is evenly distributed between the sexes. According to various studies, approximately 20–35 % of infertility cases are female, 20–30 % are male, 25–40 % are due to combined problems in both parts and 10–20 % have unexplained infertility without an identifiable cause. The type of unexplained infertility is known as idiopathic(Reference Bunkar, Pathak and Lohiya7–9). While 80 % of cases that pertain to infertility may be linked to circumstances like endometriosis or polycystic ovary syndrome (PCOS), 20 % remain non-explained. The high incidence of infertility may depend on many parameters, e.g. age, environment, lifestyle alongside health status. In light of all the aforementioned reasons, the present study will attempt to concentrate on the epigenetic dimensions of infertility in both men and women and the risk factors linked to nutrition.
As it has been mentioned in earlier parts of the paper, female fertility is the determinant of the gender role in society in addition to being a biological fact, which constitutes a constraint for specific groups such as relatively elder women. Being older than 35 years of age indeed increases infertility risk(Reference Sharma, Biedenharn and Fedor10). As for males, men's testosterone levels decline with age, and sperm motility and semen volume also decrease after the age of 35. Moreover, DNA damage in sperm increases significantly after the age of 40, and both the motility and viability of sperm are reduced(Reference Varshini, Srinag and Kalthur11). The changes are called epigenetic marks; DNA methylation, remodelling of chromatin, histone tail modifications apart from mechanisms related to non-coding RNAs(Reference Gunes and Esteves12). This takes over an eminent function in the monitoring of cellular processes such as gene expression levels, DNA–protein interactions, differentiation at cellular level, embryogenesis, aside from genomic imprinting(Reference Stotz13,Reference Das, Parbin and Pradhan14) . Genomic imprinting, a phenomenon of epigenetic gene marking emerging in the germline, results in parental-specific occurrence of a tiny gene subset of mammals. Imprinting indeed owns a significant effect over normal mammalian development, fetal growth, metabolism as well as adult behaviour. Epigenetic marks of parental origin are structured when male and female gametogenesis take place, transferred over to the zygote via fertilisation, sustained during the course of development as well as adulthood, are deleted in primitive germ cells prior to the formation of novel marks(Reference Li and Sasaki15). As is known the epigenome alone does not encode genetic information, but is responsible for the expression of genes, does not control intergenerational inheritance, but can replicate itself from cell to cell. Epigenetic changes in gametes are likened to changes that occur in the four seasons of life, and gamete development can be evaluated in four stages: embryonic development, the process up to puberty and sexual maturity, adulthood and the aging process. This journey of gametes is full of epigenetic changes(Reference Wasserzug-Pash and Klutstein16). Epigenetic modification can be transmitted relatively stably during the process of cell proliferation. In recent years, it is thought that changing gene expressions through epigenetic mechanisms may cause several health problems(Reference Zhu, Cao and Li17).
Methods
A review of the line of the literature was conducted prior to June 2021 through the determined websites, i.e. MEDLINE, Embase, Web of Science, www.ClinicalTrials.gov, Cochrane Central, PubMed, Google Scholar, Science Direct and the WHO. The targeted papers were clustered referring to the existing information using some keywords: ‘infertility, epigenetics, genome, DNA sequence modification, gene expression, diet and infertility, dietary habits, semen parameters, dietary patterns, foods, nutrients, fertility and infertility’. The keyword combinations are resorted to again through the names of infertility, epigenetics and nutrient in family names. Sub-references of the articles identified relying on keywords were sought and these were scrutinised too. In reserach on infertility, epigenetics and nutrients, animal studies together with clinical human studies were explored. The said reviews as well as research meta-analyses constitute the framework of the current research.
Epigenetic mechanisms and infertility
There are more than 200 cell types of different sort in the human body and each of these types possess the same copy of the genome. Howbeit, distinctive types of cells form different gene series that depend upon epigenetic regulation. Epigenetics examines mitotic and/or meiotic hereditary changes which influence gene expression with the lack of DNA sequence modification. The epigenome is thought to function as the second dimension of the DNA sequence that is highly effective in maintaining patterns that belong to cell type-specific gene expression(Reference Gunes and Esteves12).
Since a cell's epigenome has great plasticity and can be reprogrammed, epigenetic modifications dynamically and reversibly control gene expression. Epigenetic reprogramming alters the fate of cells throughout development and adulthood. It is noteworthy that environmental factors play an important role in the creation and maintenance of epigenetic marks(Reference Eiras, Pinheiro and Romcy18).
Male infertility
Epigenetic processes are influential in male fertilisation potential as well as sperm function. The appropriate functioning pertaining to epigenetic mechanisms, namely, ncRNAs DNA methylation, chromatin remodelling and histone tail modifications during gonadal as well as spermatogenesis development is essential for normal sperm production and function(Reference Gunes and Esteves12). Speaking of settings of clinical sort, semen analysis is commonly used to diagnose the trend of male-related infertility, to wit, DNA fragmentation analysis as well as microscopic examination, which are not sufficient to explain all events. However, spermiograms belonging to infertile patients’ normozoospermic cannot be distinguished from those of fertile individuals, which is often insufficient for the diagnosis of unexplained male infertility (UMI). Nevertheless, recent advances in sequencing technologies are promising as they can help us identify why some couples experience idiopathic infertility(Reference McSwiggin and O'Doherty19). Remodelling of chromatin, residual histone alterations alongside DNA methylation reflect the key epigenetic shifts that occur at the sperm RNAs, and level of sperm seems to play an important role too(Reference Giacone, Cannarella and Mongioì20). Methylenetetrahydrofolate reductase (MTHFR) enzyme activity and its product S-adenosylmethionine (SAM) play a role in sperm evolution, morphology and motility. It has been observed that MTHFR enzyme is an enzyme involved in folic acid metabolism and its activity may be an important factor in spermatogenesis(Reference Baranizadeh, Bahmanzadeh and Tavilani21). In a study, it was determined that MTHFR hypermethylation gene promoter occurs often in sperm obtained from infertile individuals, and it is relatively more common in males with a history of spontaneous abortion than in men without a history of spontaneous abortion, and it oftentimes impact the entire population of sperm. This result indicates a new male parameter linked with spontaneous abortion infertility. For this reason, MTHFR hypermethylation gene promoter appears to emerge as a new putative risk factor in the spontaneous abortion aetiology(Reference Rotondo, Bosi and Bazzan22). In another study, abnormal methylation was found within the gene promoters related to imprinting, spermatogenesis as well as defense system of antioxidant sort in the sperm of patients having impaired sperm DNA integrity status(Reference Song, Wang and Chen23).
Female infertility
According to the results of a study, it is known that epigenetic deviation, which is an eminent factor of biological sort, causes diseases and gene dysregulation. It has been shown that abnormal expression of the HomeoboxA10 (HOXA10) gene and constructions of epigenetic sort are effective in the pathophysiology of endometriosis(Reference Samadieh, Favaedi and Ramezanali24). A fair number of studies like the work of Taylor and Gui have also reported that the body growth regulator HOXA10 gene shows aberrations within the endometrium of females having endometriosis. Located on chromosome 7p15.2, this master regulatory gene is the member of a larger family pertaining to DNA-binding transcription parameteres sharing an utterly conserved 183-nucleotide sequence encoding a 61 amino acid homeodomain. The HOXA10 homeobox is known to control uterine organogenesis throughout the development of the embryo as well as endometrial differentiation of functional sort in adults(Reference Taylor, Arici and Olive25,Reference Gui, Zhang and Yuan26) . Expression of the HOXA10 gene belonging to fertile women who are healthy individuals is cycle dependent. Levels of HOXA10 messenger RNA (mRNA) boost significantly during the stage pertaining to mid-secretory, a condition that coincides with implantation of embryo, peak differentiation at histological level as well as elevated systemic estrogen along with levels of progesterone(Reference Samadieh, Favaedi and Ramezanali24). Expression at higher levels pertaining to HOXA10 in the endometrium is indispensable for the decidual transformation of endometrial stromal cells owing to implantation of embryo. A defect occurring in the expression of HOXA10 and its regulation which results in miscarriages of recurrent sort as well as infertility yield impaired implantation and decidualisation(Reference Godbole, Suman and Malik27).
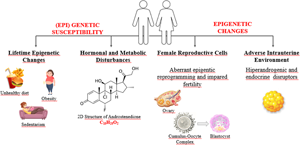
Most infertility pertaining to women is caused by PCOS, endometriosis as well as infertility of non-explained being(Reference Pisarska, Chan and Lawrenson28). PCOS is among the main reasons behind women infertility worldwide. We know that susceptibility to PCOS can be inherited not only by genetic alleles but also by epigenetic changes and developmental programming(Reference Stener-Victorin and Deng29). Environmental factors caused by hormonal and metabolic disturbances bring about (epi)genetic susceptibility to the development of PCOS throughout life. PCOS development and clinical manifestations are directly influenced by hormonal and environmental changes, so epigenetic alterations might also be influential in PCOS outcomes(Reference Eiras, Pinheiro and Romcy18).
DNA methylation
Methylation of DNA is an epigenetic marker that is frequently inquired into. This alteration of epigenetic sort is required for the development of women and men gametogenesis. Within the mammalian genome, DNA methylation emerges mainly in the frame of guanine (CpG dinucleotides) and cytosine at the fifth position pertaining to the cytosine bases. This is called 5-methylcytosine (5mc)(Reference McSwiggin and O'Doherty19). These dinucleotides are defined as methylated regions in a different fashion (a.k.a. DMRs (distinct methylated regions)), which are usually located closer to regions that are known as gene regulatory like the promoter. DNA methylation covers the methylation pertaining to non-imprinted as well as to those that are imprinted, methylation that is pertinent to elements of repetitive sort, and DNA methylation at global levels(Reference Giacone, Cannarella and Mongioì20).
Methylation of cytosine at DMRs inhibits the process that pertains to the binding of transcription factors to the locations that are considered regulatory towards the relevant genes, verging on silencing or transcriptional inactivation. On the contrary, hypomethylation of regulatory regions is attributed to enhanced gene expression(Reference Gunes and Esteves12). DNA methyltransferases (DNMTs) are named DNMT3A, DNMT1, DNMT3L and DNMT3B(Reference Eden and Cedar30). One of the enzymes that maintain DNA methylation throughout DNA replication is DNMT1. The lack of DNMT1 results in aberrations throughout spermatogenesis as well as in the loss of methylation chiefly in imprinted genes of paternal sort. The enzymes DNMT3B, DNMT3L, along with DNMT3A take part in the DNA methylation throughout germ cell development at the embryonic phase. All of the DNMTs are required for appropriate spermatogenesis to occur(Reference Cheng, Chen and Zhang31).
Sperm DNA methylation is linked to sperm changes as well as to infertility. Genes frequently linked with male infertility contain defects pertaining to DNA methylation of the imprinting genes mesoderm-specific transcript (MEST) alongside H19 as well as those of non-imprinting sort MTHFR. Methylation regulates expression in relation to imprinted genes through a pivotal process, which causes an occurrence of the allele pertaining to maternal or paternal side. Once maternal/paternal alleles are demethylated with fertilisation, genes that are imprinted retain methyl markings that pertain to the parental genome(Reference Rotondo, Lanzillotti and Mazziotta32).
Imprinted genes indicate parent-specific activity and are functionally haploid. Therefore, they become vulnerable to epigenetic dysregulation. To put it more specifically, both maternal and paternal alleles are subject to demethylation following the fertilisation stage. After that, reprogramming of genetic sort takes place within the embryo, consisting of new specific methylations. Genes that are imprinted get isolated from reprogramming of epigenetic sort following the fertilisation making transmission of abnormal methylation patterns to the relevant offspring possible(Reference Giacone, Cannarella and Mongioì20). Rasgrfl, Igf2/H19, Zdbf2 and Dlk-Gtl2 are genes that are paternally methylated within spermatozoa. The Model H19 gene is baled to encode a 29k protein as well as a cytoplasmic RNA of untranslated sort participating in the transport/synthesis and RNA processing pertaining to a protein. H19 DMR is not methylated on the allele that is maternal. For this reason, the expression of H19 is made possible and insulin-like growth factor II (IGF2) gene becomes suppressed. Yet, methylation pertaining to the H19 DMR allele of paternal sort blocks IGF2 gene expression(Reference Gunes and Esteves12). In normozoospermic males, H19 gene methylation gets linked to reactive oxygen species (ROS) levels as well as factors that pertain to semen. According to a meta-analysis study analysing aberrations of sperm DNA methylation pertaining to imprinted genes, MEST, and levels of small nuclear ribonucleoprotein polypeptide N (SNRPN) NAME DMR methylation are considerably higher in idiopathic infertile males when compared to fertile males. H19 DMR methylation levels were found to be lower in infertile men than in fertile men(Reference Gunes and Esteves12,Reference Esteves, Santi and Simoni33) . According to this, hypermethylation of the SNRPN and hypomethylation pertaining to the H19 imprinted control region (ICR) are associated with infertility. The risk of this relationship increases with the habit of smoking. Furthermore, drinking alcohol is ascribed to DNA methylation within regions of regulatory sort pertaining to the imprinted gene H19 in human and mouse spermatozoa. However, the influence over alcohol-induced DNA methylation on fertility should be investigated further(Reference McSwiggin and O'Doherty19,Reference Esteves, Santi and Simoni33) . A global DNA hypomethylation occurs with ARTs. Moreover, risk of boosted sort that pertains to imprinted disorders has been recorded following the procedures in question. This has led to the emergence of the argument that ART might lead to a loss in methylation. In a study, a global DNA methylation that is considerably distinctive was identified within sperm obtained from individuals having oligo-astheno-teratozoospermia (OAT) when compared to the controls and showed the said epigenetic abnormalities can reduce human fertility(Reference Giacone, Cannarella and Mongioì20).
Chromatin reorganisation
By the rearrangement of sperm chromatin, spermatozoa pack a large amount of DNA within a minute nucleus. Protamines are known as small proteins that are unique to spermatozoa. Thanks to the existence of the passage pertaining to protamines through histones, sperm DNA occupies smaller space within the nucleus. Thusly, it causes a condensation that is pertinent to the sperm nuclei which is of tighter sort supporting the increase in sperm motility. What is more, protamination prevents the sperm genome from degradation as well as from oxidation and molecules of harmful sort found within the women reproductive system(Reference Gunes and Esteves12). In the final phases pertaining to male gametogenesis (spermiogenesis), motile spermatozoa emerge from haploid round spermatids. Chromatin gets subject to a drastic remodelling throughout which almost all of (90–95 %) of the H2A, H2B, H3 and H4 (nucleosomal core histones) are replaced by transitional proteins first and by protamines afterwards(Reference McSwiggin and O'Doherty19).
Spermatozoa are known to be cells of excessively specialised sort. Through the course of spermatogenesis, almost all of the chromatin histones (90–95 %) are exchanged with protamines, which are nuclear proteins that are tiny and arginine-rich. The said process occurring, which is expected for spermatozoa, provides considerable DNA compaction, reduced vulnerability towards outside factors and constitutes a gene silencing mechanism. In the earlier phases of protamination, acetylation of histones increases first, which supports enzyme DNA topoisomerase action, suceeded by the replacement of histones having transition proteins (TP1 and TP2). The said DNA-binding proteins trigger the removal as well as the subsequent replacement pertaining to histones by equally expressed protamine 1 (P1) and protamine 2 (P2) (Reference Gunes and Esteves12).
DNA organisation at three levels may be observed after sperm chromatin protamination: (1) toroidal structures shaped by protamines (90–95 %), (2) nucleosomes (5 %−10 %) taking part in the chief stages of the development of embryo alongside (3) the so-called matrix binding sites. DNA segments do not contain toroidal structures or nucleosomes. MARs (Matrix Attachment Regions) warrant structural support to chromatin act as promoters in the paternal pronuclear formation after fertilisation and contribute to normal embryogenesis. Consequently, scientific evidence has shown abnormalities pertaining to protamine content can impact epigenetic information transmitted by DNA of paternal sort. Accordingly, the state of sperm protamination also affects the results of ART(Reference Giacone, Cannarella and Mongioì20).
Histone alterations
Modifications of histone negatively or in a positive fashion affect the binding of regulatory factors to DNA, resulting in diminished or escalated activity pertinent to gene and expression. Specific modifications, e.g. acetylation pertaining to H3 and H4, methylation that pertains to H3K4, as well as the ubiquitination belonging to H2B increase gene expression within testicular tissue. On the contrary, methylation pertaining to H3K27 along with H3K9 and ubiquitination pertaining to H2A result in the silencing of gene expression. It has been suggested that H3K4 and H3K27 methylation stimulate the inactivation as well as the activation of gene expression(Reference Giacone, Cannarella and Mongioì20,Reference La Spina, Romanato and Brugo-Olmedo34,Reference Yuen, Bush and Barrilleaux35) . Histones that are retained are visible in gene clusters of imprinted sort and therefore protamination as well as changes within residual histones may be the common cause of paternal infertility. Correspondingly, research conducted on 291 ART cycles identified the function pertaining to histone-protamine ratio (HPR) on embryonic development and ART outcomes. The blastocyst formation rate for HPR, ranging from 6 to 26 %, was considerably higher (87⋅8 %) than what was achieved through HPR >6 % (74⋅6 %) or <6 % (71⋅2 %). Thereupon, HPR appears to have an effect on embryo development. Based on this evidence, protamine-free sperm apart from residual histone abnormalities need to be used in programmes of ART(Reference Giacone, Cannarella and Mongioì20).
Sperm-derived coding and non-coding RNA molecules
Studies have shifted more towards elucidating the functions of these RNAs. Albeit transcriptionally quiescent, sperm are indicated to involve non-coding (ncRNA) and coding (mRNA). RNA molecules that have been declared as essential for epigenetic inheritance in a wide spectrum, earlier development as well as spermatogenesis. Sperm carry thousands of different RNAs, encompassing non-coding (ncRNA) (antisense RNA, tiny interfering RNA (siRNA), micro-RNA (miRNA), long non-coding RNA (lncRNA), together with piwi-interacting RNA (piRNA)) alongside coding (mRNA) RNAs, and through different mechanisms, it takes part in the modulation of gene expression via interrupting mRNA translation(Reference Giacone, Cannarella and Mongioì20). Analysis of transcriptomic sort pertaining to sperm from males with idiopathic infertility (normozoospermic), asthenozoospermia (reduced motility) and known fertility spotted diverse profiles of RNA among patient cohorts and emphasised the potential role belonging to the molecules in paternal fertility(Reference McSwiggin and O'Doherty19). Environmental toxicants have been shown to affect testicular disease and the epigenetic transgenerational inheritance of male infertility. It involves epigenetic changes in the germline (e.g. sperm) to affect the epigenome and transcriptome of early embryonic stem cells. As the male population has a decrease in sperm count and a dramatic increase in infertility, observations suggest that testicular disease may be an important component of male infertility aetiology of promoting epigenetic transgenerational inheritance(Reference Sadler-Riggleman, Klukovich and Nilsson36). In another study, methyloma analysis of individual blastocysts compared with fertile controls unearthed considerable changes at 6609 CpG sites linked to long-term infertility (≥60 months). In sum, long-term infertility is associated with a modified methylome in euploid blastocysts with specific concentration on regulation pertaining to genomic imprinting, which is compared to aided reproductive technologies alone(Reference Denomme, Haywood and McCallie37). H19 also encodes for non-coding RNA(Reference Li, Hao and Wang38).
Treatments related to genetics and epigenetics of infertility
The use of ARTs covers IVF. Other treatments of non-IVF fertility (NIFTs) sort, encompassing controlled ovarian stimulation and ovulation induction with intrauterine insemination, provide 4⋅6 % of live births(Reference Pisarska, Chan and Lawrenson28,Reference Sunderam, Kissin and Crawford39) . Pregnancy outcomes of adverse nature are ascribed to the usage of ART. A meta-analysis showed that the risks of pregnancy-induced hypertension, placenta previa, placental abruption, gestational diabetes mellitus, preterm birth, postpartum haemorrhage, small for gestational age, low birth weight as well as perinatal mortality are increased. Infertility of maternal sort derives from several aetiologies, which may impact subsequent placentation as well as implantation, resulting in outcomes of adverse sort such as PCOS, unexplained infertility, endometriosis, along with placental dysfunction. PCOS is responsible for 0⋅70 % of all ovulatory dysfunction cases accounting for 27 % of infertility reportings. PCOS is a disorder of endocrine metabolic sort that affects 5–15 % of females(Reference Pisarska, Chan and Lawrenson28). Even after the attainment of ovulation, females having PCOS appear to have reduced rates of cumulative pregnancy compared to subfertile populations(Reference Pisarska, Chan and Lawrenson28,Reference Schulte, Tsai and Moley40) of another sort. Endometriosis constitutes a condition depicted typically by stroma outside of the uterine cavity and endometrial glands, leading to infertility, dysmenorrhoea as well as pelvic pain. It impacts approximately 10 % of women who are at a reproductive age. As a result, it has been recommended that 25−50 % of infertile females may be with endometriosis and 30−50 % of females with endometriosis may be infertile. Epigenetic and genetic effects pertaining to infertility aetiologies alongside effects of environmental sort belonging to fertility treatments upon epigenetics affect placentation and implantation. This results in shorter as well as longer-term maternal and fetal/child outcomes that are of adverse sort(Reference Pisarska, Chan and Lawrenson28).
Single gene disorders resulting in primary sterility as well as disease are detected. That said, a smaller ratio pertaining to infertility aetiologies are contributed by these specific disorders and failed to explain many of the multifactorial causes(Reference Joshi and Chan41). More recently, single nucleotide polymorphisms (SNPs) have been ascribed to diseases that cause infertility(Reference Pisarska, Chan and Lawrenson28,Reference Telenti, Pierce and Biggs42) .
Epigenetic modifications are inherited that cannot be caused by the alterations in the DNA sequence. Diseases are impacted by environmental factors and genetic variability(Reference Li, Hao and Wang38,Reference Grimstad and Decherney43) . These non-coding mechanisms of gene regulation contain non-coding regulatory elements and DNA methylation. They regulate gene expression patterns by altering DNA accessibility and chromatin structure(Reference Pisarska, Chan and Lawrenson28). Short non-coding RNAs, containing lncRNAs as well as miRNAs, influence the overall gene expression in the transcriptome. Longer non-coding RNAs are single-stranded non-coding RNAs containing 200 nucleotides. miRNAs consist of 20–24 nucleotides which modulate gene expression via effects of post-transcriptional sort. Over 2000 miRNAs have been spotted in human beings, and they make up one-third of all of the genes found within the genome(Reference Pisarska, Chan and Lawrenson28,Reference Hammond44) . In spite of the aetiological bases of infertility, epigenetic alterations that occur on account of infertility treatment play a role in chronic longer-term diseases, involving differential methylation of genes vital for development and growth with longer-term implications pertaining to health(Reference Pisarska, Chan and Lawrenson28). It has not yet been defined if any of the said differentially altered genes are linked to the treatments or to infertility. One study highlighted that antioxidant administration to IVF children enhanced nitric oxide bioavailability and vascular response within systemic as well as pulmonary circulation(Reference Rimoldi, Sartori and Rexhaj45). The results italicise ART-induced vascular dysfunction in young individuals is reversible despite redox regulation. Hence, it is deemed important to be able to come up with a clear perspective pertaining to the factors that come into play in the occurrence of infertility treatment which can improve outcomes(Reference Pisarska, Chan and Lawrenson28) (Fig. 1).

Fig. 1. Characteristics and suggested modification of diet that adversely affects fertility.
Nutritional models that increase infertility risk
Recently, the key nutrition model pertaining to developed and developing countries has been called the Western diet. The Western diet is portrayed by simple carbohydrates, saturated and trans-fatty acids as well as by a high intake of animal protein alongside essential low unsaturated fatty acids and low dietary fibre(Reference Varlamov46). The factors affecting the quality of semen have worsened through the spread of this Western diet(Reference Danielewicz, Przybyłowicz and Przybyłowicz47). Processed meats, or as indicated in a number of sources red meat, fatty dairy products, alcohol, sweets and sweet drinks, coffee, potatoes, shortage of whole-grain products, fruits and vegetables, fish and seafood, nuts, poultry and skim dairy products weaken semen parameters and cause low fertility(Reference Salas-Huetos, Bulló and Salas-Salvadó48,Reference Giahi, Mohammadmoradi and Javidan49) .
High-fat diet together with obesity caused because of leading an unhealthy impact the nature of spermatozoa, and the development along with the health of the offspring throughout a lifespan. As a matter of fact, dietary patterns of inappropriate sort like inadequate antioxidant intake, high-energy density, also skipping meals have been witnessed in infertile males(Reference Giahi, Mohammadmoradi and Javidan49,Reference Silvestris, Lovero and Palmirotta50) . It has been perceived that lower paternal dietary folate can modify the mouse sperm epigenome and to that end is ascribed to adverse outcomes pertaining to pregnancy(Reference Lambrot, Xu and Saint-Phar51). In the systematic review and meta-analysis, relationships were recognised between the paternal folate status and sperm quality, fertility, congenital malformations placental weight(Reference Hoek, Steegers-Theunissen and Willemsen52). Improper paternal and/or maternal diet may affect the epigenetic marks of the offspring and ultimately lead to infertility(Reference Lambrot, Xu and Saint-Phar51–Reference Shukla, Chambial and Dwivedi53).
Phytoestrogens
There is some controversy regarding the effect of phytoestrogens on male reproductive health. Phytoestrogens are plant-derived compounds with estrogen-like activities. The binding affinity of phytoestrogens to estrogen receptors is 100–1000 times lower than estrogen(Reference Tremellen and Pearce54). After phytoestrogens bind to the receptors with ligands, they are transported from the cytoplasm to the nucleus, enabling the expression of specific genes. At the same time, since phytoestrogens are in steroid-like structure, they can bind to receptors on the cell surface(Reference Sirotkin and Harrath55). Due to these properties, phytoestrogens can have an effect on all processes that suppress the synthesis of sex hormone-binding globulin (SHBG) regulated by estrogens and the aromatisation of testosterone(Reference Yanagihara, Zhang and Toyohira56). Aside from effects of estrogenic sort, it has properties of antimutagenic and antioxidant nature(Reference Messina and Messina57). Also, Asian individuals have the capability to convert soy into an estrogen of non-steroidal being by gut bacteria, which is affected by genetic conditioning, diet and the composition of the gut microbiota(Reference Desmawati and Sulastri58). Existing line of research do not show soybean intake at moderate levels is linked with an elevated risk pertaining to infertility, impaired semen quality alongside decreased levels of blood testosterone. On top of these enhancement in sperm quality components was unveiled in some cases(Reference Cooper59). It is known that infertility is seen in sheep consuming high amounts of soy. Although there are studies that associate regular consumption of soy products with decreased sperm count in men and reduced fertility in women, recent studies have reported that consumption of soy products or soy supplement intake will not affect fertility in humans and may even increase the live birth rate with ART(Reference Morin60–Reference Gaskins, Nassan and Chiu63). As a result, the consumption of soy products does not adversely affect fertility and may even reduce the risk of ovulatory infertility in women(Reference Morin60,Reference Gaskins and Chavarro62,Reference Gaskins, Nassan and Chiu63) . However, prospective research investigating the impact pertaining to phytoestrogens is needed to be able to pinpoint the influence of isoflavone consumption in fertility.
Antioxidants
In the Cochrane review, in which randomised controlled studies on antioxidant supplementation were published in the treatment of infertility, it was reported that antioxidant supplementation had no benefit in increasing pregnancy or live birth rates(Reference Showell, Mackenzie-Proctor and Brown64). Still, selenium deficiency is associated with reproductive system disorders. It is reported that selenium scavenges hydrogen peroxide molecules by decreasing the production of ROS in sperm and increases glutathione peroxidase-1 activity. Daily 400IU vitamin E and 200 μg selenium supplementation for 100 d to infertile men aged 20–45 years resulted in a 52⋅6 % increase in sperm motility and morphology, and 10⋅8 % pregnancy occurred as a result of combined treatment with vitamin E and selenium(Reference Moslemi and Tavanbakhsh65). On the other hand, it is also reported that selenium supplementation alone is not effective in the treatment of infertility. For example, 200–300 mg/d of selenite supplementation, selenium-enriched yeast or high dietary selenium intake did not have a positive effect on sperm characteristics or activity, in spite of increased semen selenium concentrations(Reference Mistry, Pipkin and Redman66). Another study showed that the combination of 100 μg selenium and 1 mg vitamin A improved sperm motility in subfertile men with low selenium levels. Zinc, another antioxidant trace mineral, is necessary for sperm DNA condensation. The low zinc content of sperm chromatin is associated with male infertility. It is known that selenium and zinc positively affect sperm concentration and sperm motility(Reference Charkamyani, Khedmat and Hosseinkhani67). It has also been suggested that insufficient zinc consumption may damage antioxidant defence and be an important danger factor in oxidant release, and it will be effective in making sperm cells extremely prone to oxidative damage. In men with idiopathic asthenozoospermia or oligospermia, oral zinc supplementation has been found to positively affect sperm motility, morphology and count(Reference Yao and Mills68).
Vitamins
Vitamin B12 and folate (or folic acid) are under investigation for beneficial effects on fertility. While the effect of defects and folate deficiency in folate as well as homocysteine metabolism on neural tube defects (NTDs) has been determined the evidence for folate's effects on fertility is less clear(Reference Bibbins-Domingo, Grossman and Curry69). It is thought that folate may have beneficial effects on fertility in infertility treatment. Folate, which is involved in DNA synthesis, is a very important vitamin for gametogenesis, fertilisation and pregnancy. Thereby, folate (the dietary form) or folic acid (the synthetic form) has a critical role in reproduction(Reference Chiu, Chavarro and Souter70). Low folate intake is associated with increased anovulation. Increasing the folate stores in the body has been shown to improve oocyte quality during IVF treatment(Reference Tremellen and Pearce54). IVF cohort research in Poland discovered females taking folic acid supplements before treatment were found with better quality oocytes as well as with a higher degree of mature oocytes compared to those who did not(Reference Szymański and Kazdepka-Ziemińska71). Another study highlighted that folic acid supplementation in infertile women decreased homocysteine concentrations and increased folate concentrations in follicular fluid, which was associated with greater chances of pregnancy and better embryo quality(Reference Boxmeer, Macklon and Lindemans72). It is suggested all females of childbearing age take a folic acid supplement of 400 mcg/d and include dark green leafy vegetables which are rich sources of folate, in their diets(Reference Gaskins and Chavarro62). In a study conducted on subfertile women, it was demonstrated that the probability of getting pregnant was 16 % more in the group taking a multivitamin supplement with 400 mcg/d of folic acid compared to the group taking placebo(Reference Chiu, Chavarro and Souter70).
Insufficient intake of folate, vitamins B6 and B12, which are influential in the homocysteine pathway in the preconceptional period, is linked to fertility outcomes in partners that are of adverse sort. Insufficient intake of these vitamins can cause an increase in homocysteine levels, generating hyperhomocysteinemia. Evidently, increased homocysteine level in follicular fluid is inversely proportional to oocyte and embryo quality and worsens IVF/ICSI(Reference Tremellen and Pearce54).
Vitamins A, C and E (carotenoids, α-tocopherol, ascorbic acid) can provide antioxidant–prooxidant stability and protect the genetic unity of sperm cells by prohibiting oxidative damage in sperm DNA, increase the number of motile sperm as well as sperm count with vitamin C along with B-carotene intake. It has been reported that vitamin C supplement has a positive impact upon stress-induced infertility due to its testosterone-increasing and antioxidant effects. It is also stated that high-dose of vitamin C supplementation increases the testosterone level by affecting the hypothalamus–pituitary–testis axis(Reference Sanlier and Yorusun73). Antioxidants thought to be associated with infertility are vitamins A, C, E, zinc, selenium, glutathione, coenzyme Q10, carnitine and lycopene. In addition to their positive effects on free radicals, they are also associated with infertility(Reference Cecchino, Seli and da Motta74). There are vitamin D receptors in the ovaries, uterus, endometrium and placenta. It has been reported that the effect of vitamin D on infertility may also be by increasing the level of interleukin-6 (IL-6) which is one of the pro-inflammatory cytokines. In a study, it was found that women with both low serum vitamin D levels and high IL-6 levels had a 10⋅6 times higher risk of infertility due to tubal factors(Reference Chen, Jiao and Zhang75). It has also been pronounced that inflammatory factors such as IL-6 show a significant correlation in individuals with endometriosis complicated with infertility(Reference Wang, Ma and Song76).
It has been concluded that there is a direct correlation between semen quality and serum 25(OH) vitamin D levels in men and vitamin D receptors (VDRs) are importantly lower in infertile men compared to other men(Reference Tremellen and Pearce54). In adults, low serum vitamin D is also associated with low sperm count, and changes in sperm morphology and sperm motility(Reference Heyden and Wimalawansa77). In another study carried out on infertile women, it was figured out there existed no statistically significant difference between those with and without adequate serum 25(OH) vitamin D levels in terms of follicle-stimulating hormone (FSH) and AMH(Reference Shapiro, Darmon and Barad78). It is uttered that the beneficial effects of vitamin D on reproductive health occur not only through the VDR in germ cells but also through the ability of other organs in the male reproductive system to express VDR. Vitamin D is also required for transcellular calcium transfer from serum to epididymis, which is required for sperm motility in maturation of spermatoses(Reference Tremellen and Pearce54). Antioxidant nutritional supplements such as l-carnitine and CoQ10 can remarkably improve semen parameters. It has been stated that the use of antioxidant nutritional supplements in the treatment of male infertility may be the focus of future reports(Reference Omar, Pal and Kelly4). There are also studies claiming the opposite(Reference Rossi, Abusief and Missmer61). In another study, it was reported that micronutrient supplementation such as vitamins B6, C as well as D and E, along with folic acid, iron, selenium, and lastly iodine may have a positive effect on infertility treatment(9). Iron, copper and manganese gain a prominent function towards the development of fetus positively impacting female reproductive system. But still, both excess and deficiency of essential trace elements are ascribed to adverse pregnancy outcomes along with infertility in women(Reference Skalnaya, Tinkov and Lobanova80) (Table 1).
Conclusions and recommendations
Infertility treatment is time-consuming and costly, as well as an emotionally tiring process. Since there are some non-modifiable factors that determine the success of assisted reproductive technologies, the focus should be on optimising modifiable lifestyle parameters enhancing fertility or the likelihood of the effectiveness of treatment pertaining to infertility. Epigenetic mechanisms can be affected by numerous parameters, namely, aging, environmental influence, dietary energy as well as consumption of trans-fatty and saturated acids, intake of mono and polyunsaturated fatty acids (especially omega 3), animal protein sources, as well as nutritional and non-nutrient compounds, more or less energy intake or methionine interact with D, B12 and B6 vitamins, biotin, choline, iron, selenium, zinc, folic acid, resveratrol and/or quercetin.
Even though the epigenetic mechanisms, genes, nutrition and nutritional supplements discussed in this review have an effect on infertility and the recommended dose has not been determined yet, it has been noted that they can positively affect fertility. However, more comprehensive and longitudinal human studies investigating its relationship with male and female reproductive functions are needed.
Acknowledgements
The present study did not obtain any grants of specific sort from any funding agency belonging to the public, commercial or not-for-profit sectors.
Research idea: K. E., N. T. S. and N. S.; Design of the study: K. E. and N. S.; Drafting the manuscript: K. E., N. T. S. and N. S.; Revising it critically for important intellectual content: K. E., N. T. S. and N. S.; Final approval of the version to be published: K. E., N. T. S. and N. S.
The authors of this study declare herein that they do not have any interests of competing sort.