Published online by Cambridge University Press: 06 February 2019
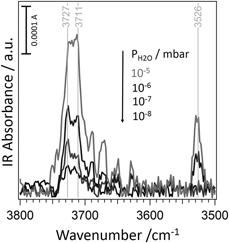
The configurations associated with the dissociative adsorption of water on a variety of low-coordinated sites of MgO(100) surfaces, including corners, steps, MgO vacancies, and kinks on 〈010〉 steps, have been studied and assigned by combining infrared spectroscopy and ab initio calculations. Three kinds of MgO powders were examined: powders of very high specific surface area prepared by chemical vapor synthesis and well-defined cubic smoke particles obtained by combustion in either 20:80 or 60:40 O2:Ar mixtures, the latter one involving less defects and smaller particles. It appears that an imperative requirement to obtain a precise characterization of the reactive behavior of defects is to keep the samples in ultra–high vacuum conditions and to control the water partial pressure finely.