Article contents
UV photo-chlorination and -bromination of single-walled carbon nanotubes
Published online by Cambridge University Press: 21 January 2014
Abstract
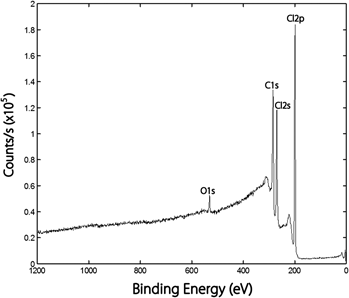
Electron-withdrawing halogen atoms are often bonded to the surface of carbon nanotubes to assist in the conversion from metallic to semiconducting properties. Single-walled carbon nanotubes (SWCNTs) were surface modified using UV photolysis with: (i) a broad band of wave lengths from approximately 250 to 400 nm having a maximum intensity at approximately 300 nm for photolysis of Cl2, (ii) low-pressure Hg lamps emitting 253.7 nm photons for photo-decomposition of HBr, and (iii) low-pressure Hg lamps emitting both 253.7 and 184.9 nm for photo-dissociation of HCl and HBr, respectively, and analyzed by x-ray photoelectron spectroscopy. Chlorine atoms adhered more readily than bromine atoms with the π-conjugation of the SWCNTs. The dominant increase with treatment was observed in the singly bonded chlorine moiety. Chlorine atoms, generated by UV photolysis of Cl2, produced a higher Cl saturation level of approximately 36 at.% than previously observed for multi-walled carbon nanotubes (13 at.%).The degree of chlorination depended on the amount of oxygen on the surface of the SWCNTs. Photo-dissociation of gaseous HCl and HBr showed lower amounts of halogenation on SWCNTs (approximately 5.8 at.% Cl and 2.5 at.% Br, respectively) than photolysis of Cl2.
- Type
- Articles
- Information
- Copyright
- Copyright © Materials Research Society 2013
References
REFERENCES
- 11
- Cited by


