Article contents
Toward tailored functionality of titania nanotube arrays: Interpretation of the magnetic-structural correlations
Published online by Cambridge University Press: 09 May 2013
Abstract
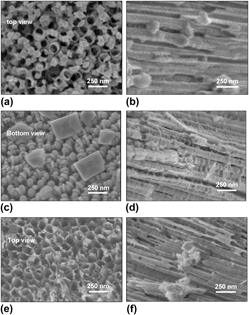
Ordered arrays of titania nanotubes (NTs) are considered as good candidates for photocatalytic applications including water splitting. Considering that the functionality of these nanostructures is influenced by their morphology, electronic and the crystallographic structure, fundamental understanding of these properties and their possible correlations can clarify the approaches toward enhanced photocatalytic efficiency. In this work, ordered arrays of titania NTs are synthesized electrochemically and are subjected to isochronal annealing treatments in various atmospheres (oxygen-rich, oxygen-deficient and reducing) to modify their morphology, crystal and electronic structure. Upon characterization of these NTs, direct correlations are found between the annealing atmosphere and the corresponding unit cell volume and the crystallite size. Furthermore, correlations between the NTs’ structure and magnetic response are observed, revealing changes in the electronic structure such as the density of states, that are in turn relevant to the functional catalytic properties of titania.
- Type
- Articles
- Information
- Copyright
- Copyright © Materials Research Society 2013
References
REFERENCES
- 6
- Cited by


