Crossref Citations
This article has been cited by the following publications. This list is generated based on data provided by
Crossref.
Lux, Alexander
Lukačová, Zuzana
Vaculík, Marek
Švubová, Renáta
Kohanová, Jana
Soukup, Milan
Martinka, Michal
and
Bokor, Boris
2020.
Silicification of Root Tissues.
Plants,
Vol. 9,
Issue. 1,
p.
111.
Wu, Xuanhao
Lee, Byeongdu
and
Jun, Young-Shin
2020.
Interfacial and Activation Energies of Environmentally Abundant Heterogeneously Nucleated Iron(III) (Hydr)oxide on Quartz.
Environmental Science & Technology,
Vol. 54,
Issue. 19,
p.
12119.
Soukup, Milan
Rodriguez Zancajo, Victor M
Kneipp, Janina
Elbaum, Rivka
and
Zhao, Qiao
2020.
Formation of root silica aggregates in sorghum is an active process of the endodermis.
Journal of Experimental Botany,
Vol. 71,
Issue. 21,
p.
6807.
Santana, Maricela
Montoya, Gonzalo
Herrera, Raúl
Hoz, Lía
Romo, Enrique
Zamora, Claudia
Wintergerst, Ana
and
Arzate, Higinio
2020.
Cemp1-p3 Peptide Promotes the Transformation of Octacalcium Phosphate into Hydroxyapatite Crystals.
Crystals,
Vol. 10,
Issue. 12,
p.
1131.
Kumar, Santosh
Natalio, Filipe
and
Elbaum, Rivka
2021.
Protein-driven biomineralization: Comparing silica formation in grass silica cells to other biomineralization processes.
Journal of Structural Biology,
Vol. 213,
Issue. 1,
p.
107665.
Dhiman, Pallavi
Rajora, Nitika
Bhardwaj, Shubham
Sudhakaran, Sreeja S.
Kumar, Amit
Raturi, Gaurav
Chakraborty, Koushik
Gupta, Om Prakash
Devanna, B.N.
Tripathi, Durgesh Kumar
and
Deshmukh, Rupesh
2021.
Fascinating role of silicon to combat salinity stress in plants: An updated overview.
Plant Physiology and Biochemistry,
Vol. 162,
Issue. ,
p.
110.
MOORE, KELSEY R.
PRESENT, THEODORE M.
PAVIA, FRANK
GROTZINGER, JOHN P.
HOLLIS, JOSEPH RAZZELL
SHARMA, SUNANDA
FLANNERY, DAVID
BOSAK, TANJA
TUITE, MICHAEL
KNOLL, ANDREW H.
and
WILLIFORD, KENNETH
2022.
BIOSIGNATURE PRESERVATION AIDED BY ORGANIC-CATION INTERACTIONS IN PROTEROZOIC TIDAL ENVIRONMENTS.
PALAIOS,
Vol. 37,
Issue. 9,
p.
486.
Huang, Liming
Tang, Luping
Gu, Haitao
Li, Zhen
and
Yang, Zhenghong
2022.
New insights into the reaction of tricalcium silicate (C3S) with solutions to the end of the induction period.
Cement and Concrete Research,
Vol. 152,
Issue. ,
p.
106688.
Sheng, Huachun
Li, Ying
Feng, Jingqiu
and
Liu, Yuan
2023.
Regulation of thermodynamics and kinetics of silica nucleation during the silicification process in higher plants.
Plant Physiology and Biochemistry,
Vol. 198,
Issue. ,
p.
107674.
Picker, Andreas
Nicoleau, Luc
Nonat, André
Labbez, Christophe
and
Cölfen, Helmut
2023.
Influence of polymers on the nucleation of calcium silicate hydrates.
Cement and Concrete Research,
Vol. 174,
Issue. ,
p.
107329.
Tong, Tiezheng
Liu, Xitong
Li, Tianshu
Park, Shinyun
and
Anger, Bridget
2023.
A Tale of Two Foulants: The Coupling of Organic Fouling and Mineral Scaling in Membrane Desalination.
Environmental Science & Technology,
Vol. 57,
Issue. 18,
p.
7129.
Ayieko, Vincent Otieno
Cohen, Lilian
Diehn, Sabrina
Goobes, Gil
and
Elbaum, Rivka
2023.
Siliplant1 B-domain precipitates silica spheres, aggregates, or gel, depending on Si-precursor to peptide ratios.
Colloids and Surfaces B: Biointerfaces,
Vol. 232,
Issue. ,
p.
113582.
Palakurthy, Srinath
Houben, Lothar
Elbaum, Michael
and
Elbaum, Rivka
2024.
Silica Biomineralization with Lignin Involves Si–O–C Bonds That Stabilize Radicals.
Biomacromolecules,
Vol. 25,
Issue. 6,
p.
3409.
Chen, Wei
Shi, Xiaoyu
Cai, Jun
and
Yang, Hu
2024.
Insight into the synergistic behaviors of a hydroxyethyl cellulose-based graft copolymer in the mitigation of silica scaling: Combined static and reverse osmosis tests.
Journal of Membrane Science,
Vol. 689,
Issue. ,
p.
122179.
McCutchin, Christina A.
Edgar, Kevin J.
Chen, Chun-Long
and
Dove, Patricia M.
2025.
Silica–Biomacromolecule Interactions: Toward a Mechanistic Understanding of Silicification.
Biomacromolecules,
Vol. 26,
Issue. 1,
p.
43.
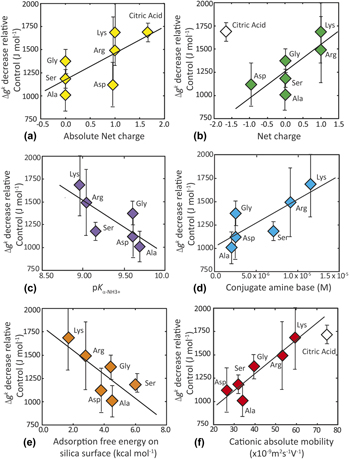
 $\left( {{K_{\alpha {\rm{ ‐ N}}{{\rm{H}}_3}^ {\bf{+}} }}} \right)$ group and thus the abundance of the conjugate base. Citric acid, lacking amine groups, promotes the greatest rate enhancement, thus demonstrating the role(s) of additional kinetic factors in promoting nucleation rate. Catalytic activity correlates with multiple physical and chemical properties of the organic acids.
$\left( {{K_{\alpha {\rm{ ‐ N}}{{\rm{H}}_3}^ {\bf{+}} }}} \right)$ group and thus the abundance of the conjugate base. Citric acid, lacking amine groups, promotes the greatest rate enhancement, thus demonstrating the role(s) of additional kinetic factors in promoting nucleation rate. Catalytic activity correlates with multiple physical and chemical properties of the organic acids.