Published online by Cambridge University Press: 29 August 2012
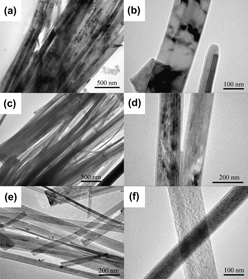
TiO2@C core–shell nanostructures with various crystal structures of TiO2-B, anatase, and rutile were successfully synthesized by a simple hydrothermal process and postheat treatments. As-synthesized precursor hydrogen titanate@carbonaceous nanoribbons transformed into TiO2-B@C nanoribbons at 400 °C and further transformed into anatase and rutile TiO2@C nanoribbons at 700 and 800 °C, respectively. The morphology of nanoribbons can be retained up to 800 °C. The transformation temperature (800 °C) from anatase to rutile phase is lower than that of TiO2 nanofibers without carbon layers and anatase TiO2@C nanoparticles. These results show that the carbon shell plays important roles in promoting the phase transition from anatase to rutile phase and protecting the nanoribbon-like morphology. The formation mechanism of the TiO2@C core–shell nanostructures with various crystal structures was discussed.