Published online by Cambridge University Press: 02 October 2014
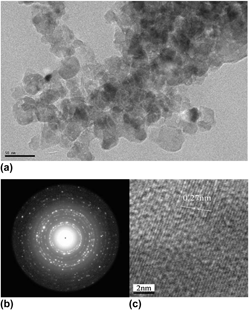
Nanosized Eu2+-doped AlN phosphor was successfully synthesized by a metal-organic precursor method for the first time. Aluminum and europium chlorides were simultaneously reacted with triethylamine in acetonitrile media to yield solid precipitates, which were transformed into nanosized AlN:Eu2+ phosphor powders upon calcination in an ammonia gas atmosphere. The possible reaction mechanism was proposed, which is in good agreement with the experimental results. The direct formation of Al–N bonds through a coordination reaction in solution is a key factor in the formation of well-crystallized AlN:Eu2+ grains at a moderately low temperature (1200 °C), which significantly suppresses abnormal grain growth and favors the formation of nanocrystalline (∼15 nm) particles with a homogeneous particle size distribution. Due to the homogeneous distribution of a relative high amount of Eu incorporation (2 wt%), the nanophosphors were effectively excited by UV light and featured an intense green emission band with a peak at 506 nm.