Published online by Cambridge University Press: 01 January 2011
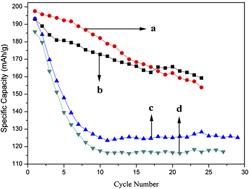
Shape control of nanocrystals has become an indispensable part in material research, such as developing new battery raw materials and synthesizing high activity catalysts. In this work, one-dimensional LiV3O8 nanorods have been fabricated by high temperature solid-state reaction using V2O5 nanowires as precursors obtained via a hydrothermal method. The as-prepared LiV3O8 nanorods were characterized by x-ray diffraction, transmission electron microscopy, scanning electron microscopy, and galvanostatic tests, compared with LiV3O8 samples synthesized by the traditional one-step solid-state method. The results show that LiV3O8 nanorods exhibited better electrochemical performance than those synthesized by the traditional method, indicating that a different shape will lead to huge distinctions in electrochemical properties. This work demonstrates that Li-insertion/deintercalation dynamics might be crystal morphology-sensitive.