Article contents
Synthesis of hollow porous nanospheres of hydroxyl titanium oxalate and their topotactic conversion to anatase titania
Published online by Cambridge University Press: 03 June 2011
Abstract
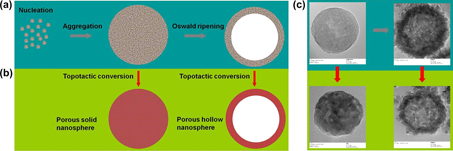
A one-step wet chemistry route has been explored to synthesize hollow hydroxyl titanium oxalate nanoscale spheres under mild experimental conditions. The hollow spheres were ∼200 nm in diameter, with a shell thickness of ∼30 nm. The nanospheres were formed by smaller aggregated colloidal subunits. The influence of temperature and solvent on the structure of the nanospheres was investigated. The formation of hollow interiors in the nanospheres may be rationalized by Ostwald ripening mechanism. Simple thermal treatment topotactically transformed the chemical composition into anatase TiO2. The high-order hollow porous spherical structure was preserved, with smaller crystalline anatase TiO2 nanoparticles as building units. Dense hydroxyl titanium oxalate nanospheres and their corresponding non-hollow porous anatase TiO2 nanospheres were also successfully achieved in suitable reaction conditions. The method and procedure reported herein may be extended in principle for the fabrication of other functional materials.
Keywords
- Type
- Articles
- Information
- Copyright
- Copyright © Materials Research Society 2011
References
REFERENCES
- 8
- Cited by


