Article contents
Synthesis and luminescence of Sr2Ta2O7:Pr3+: a novel blue emission, long persistent phosphor
Published online by Cambridge University Press: 03 November 2016
Abstract
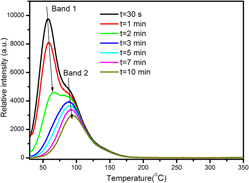
In this paper, a novel afterglow phosphor based on praseodymium ion doped Sr2Ta2O7 was synthesized successfully by solid-state reaction in the ambient atmosphere. The photoluminescence, afterglow, afterglow decay, and thermoluminescence (TL) properties were investigated in detail. The dependence of photoluminescence properties and long afterglow (LAG) performances on Pr3+ contents were investigated systematically. The optimal concentrations of Pr3+ ions for the best photoluminescence and LAG properties were experimentally to be 2 mol% and 0.5 mol%, respectively. Pr3+ exhibits prominent red emission in most reports, which derives from the 1D2 → 3H4 transition. However, the predominant blue emission locating at ∼489 and ∼507 nm coming from 3P0,1 → 3H4 transitions were observed in praseodymium ion-doped Sr2Ta2O7. Based on TL measurements, the trapping and de-trapping processes of charge carriers between shallower and deep traps were illustrated. A model was proposed on the basis of experimental results to explain the mechanisms of photoluminescence and LAG.
- Type
- Articles
- Information
- Copyright
- Copyright © Materials Research Society 2016
References
REFERENCES
- 5
- Cited by



