Published online by Cambridge University Press: 16 May 2011
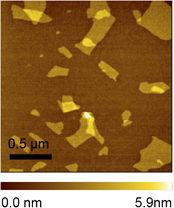
Here, we describe the synthesis of novel titania nanosheets controllably doped with binary transition metal ions and their layer-by-layer self-assembly. The tailored Mn and Fe doping in exfoliated Ti0.6Mnx/2Fe(0.8-x)/2O2 (x = 0.0–0.4) nanosheets is achieved by systematically changing the molar ratio of Mn/Fe in K0.8Ti1.2MnxFe0.8-xO4 using a codoping strategy. The protonated layered crystals exhibit a delaminated behavior in the tetrabutylammonium hydroxide solution and are exfoliated into colloidal single sheets, which are characterized by a large lateral size and a thickness in molecular dimension. The resulted nanosheets are able to be layer-by-layer deposited with oppositely charged polymers into a composite organic/inorganic system.