Published online by Cambridge University Press: 11 October 2019
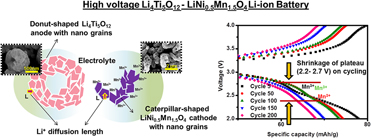
The design of high energy Li-ion batteries (LIBs) by coupling high voltage LiNi0.5Mn1.5O4 (LNMO) cathode and Li4Ti5O12 (LTO) anode ensures effective and safe energy-storage. LTO–LNMO full-cells (FCs) with difference in electrode grain sizes and presence of excess Mn3+ in cathode were studied using micron-sized commercial LTO, nanostructured LTO donuts (LTOd), P4332 LNMO nanopowders, and nanostructured Fd3m LNMO caterpillars (LNMOcplr). Among the studied FCs, LTOd–LNMOcplr was detected with a stable capacity of 69 mA h/g (1C rate), 99% coulombic efficiency, and 87% capacity retention under 200 cycles of continuous charge–discharge studies. The superior electrochemical performance observed in LTOd–LNMOcplr FC was due to the low charge transfer resistance, which is corroborated to the effect of grain sizes and the longer retention of Mn3+ in the electrodes. An effective and simple FC design incorporating both nanostructuring and in situ conductivity in electrode materials would aid in developing future high-performance LIBs.
Present Address: Department of Chemical and Bio-molecular Engineering, Rice University, Houston, Texas, USA.