Published online by Cambridge University Press: 27 August 2019
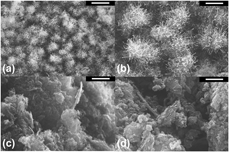
Using ethanol adsorption calorimetry, the surface energetics of two carbon substrates and two products in microwave-assisted carbon nanotube (CNT) growth was studied. In this study, the ethanol adsorption enthalpies of the two graphene-based samples at 25 °C were measured successfully. Specifically, the near-zero differential enthalpies of ethanol adsorption are −75.7 kJ/mol for graphene and −63.4 kJ/mol for CNT-grafted graphene. Subsequently, the differential enthalpy curve of each sample becomes less exothermic until reaching a plateau, −55.8 kJ/mol for graphene and −49.7 kJ/mol for CNT-grafted graphene, suggesting favorable adsorbate–adsorbent binding. Moreover, the authors interpreted and discussed the partial molar entropy and chemical potential of adsorption as the ethanol surface coverage (loading) increases. Due to the low surface areas of carbon black–based samples, adsorption calorimetry could not be performed. This model study demonstrates that using adsorption calorimetry as a fundamental tool and ethanol as the molecular probe, the overall surface energetics of high–surface area carbon materials can be estimated.
These authors contributed equally to this work.
This author was an editor of this journal during the review and decision stage. For the JMR policy on review and publication of manuscripts authored by editors, please refer to http://www.mrs.org/editor-manuscripts/.