Published online by Cambridge University Press: 07 February 2020
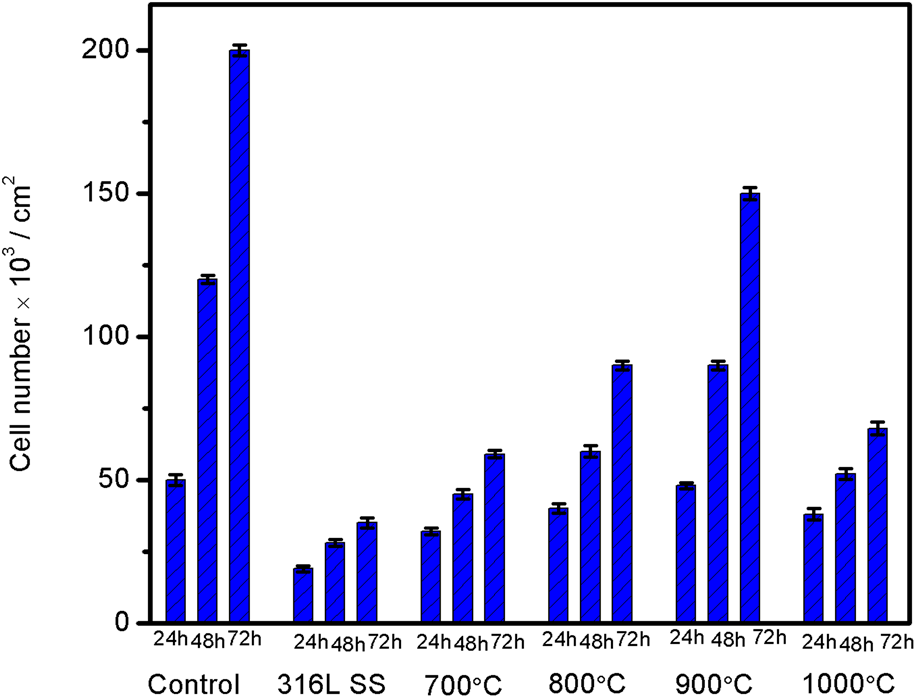
The present work is mainly accentuated to improve corrosion resistance performance, adhesion strength, biocompatibility, and cell proliferation of metallic implants. Novel nano triphasic bioceramic composite coating was achieved on 316L SS by the electrophoretic deposition process followed by vacuum sintering. The optimized potential for composite coating on 316L SS was found to be 30 V and 1 min. All the composite coated samples were sintered in a vacuum furnace at various sintering temperature starting from 700 °C to 1000 °C for 1 h. The coated samples were thoroughly characterized in terms of crystallinity, morphology, and surface roughness by XRD, FESEM with EDX, and profilometer studies, respectively. In addition, the coated samples were mechanically characterized using a tap adhesion and Vickers microhardness test. Corrosion performance of the coated sample was characterized by electrochemical studies in Hank's solution. The in vitro cytotoxicity studies for cell viability and cell proliferation was carried out using MC3T3-E1 osteoblast cells. These studies revealed an enhanced cell attachment and proliferation on the composite-coated sample than the uncoated sample, which controlled the discharge of metal ions from the metal surface into the biological system.