Published online by Cambridge University Press: 26 February 2016
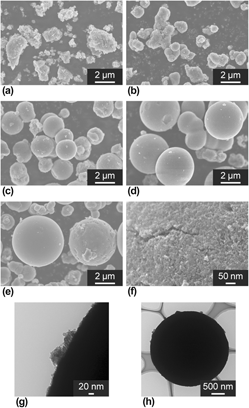
Although there are numerous reports on the synthesis of spherical materials, the development of new approaches remains important for theory construction to realize tailor-made synthesis of spherical materials. Herein, we report the synthesis of polydispersed spherical particles of H2Ti2O5 intercalated with a polyethyleneamine, such as an ethylenediamine, on the basis of a solvothermal treatment using concentrated polyethyleneamine aqueous solutions. The diameter of the micrometer-sized spheres enlarged with increasing amine concentration in the reaction solution. It was speculated that high ionic strength caused the self-assembly of polyethyleneamine-intercalated H2Ti2O5, resulting in the formation of spherical agglomerates. The spheres had a specific a surface area of 200 m2 g−1 and approximately 5 nm pores, and these values were controlled by amine concentration and treatment time. Conversion to single phase anatase and rutile without changes in spherical morphology was achieved by heat treatment. The present approach may assist with the design morphology of agglomerates.