I. INTRODUCTION
In recent years, flexible or bendable energy storage and conversion systems, which are designed to be portable, lightweight, bendable and even wearable, have attracted tremendous attention due to their mechanical flexibility and high energy density.Reference Armand and Tarascon1–Reference Chen, Xin, Zhou, Ma, Zhou and Qi3 Among various energy-storage devices, lithium-ion batteries (LIBs) and electrochemical capacitors (ECs) are two of the most promising candidates because of their high energy density, large power density and excellent stability.Reference Arico, Bruce, Scrosati, Tarascon and Van Schalkwijk4 Due to the excellent properties of flexible devices, LIBs and ECs, a combination of flexible electrodes with LIBs and ECs has attracted much more interest as an important branch of bendable electronic systems.
However, the bottlenecks of transforming the traditional batteries and capacitors into flexible energy storage devices lie in the preparation methods, assembly process, and the selection of proper electrolytes. Conventional LIBs and ECs usually consist of a carbon-based anode, a transition metal oxides-based cathode, a polymer separator and organic liquid electrolyte. However, there are several disadvantages for the traditional methods. In these batteries, relatively heavy metal foils are always used as both conductive substrate and structural support with slurry of active materials, binders and conductive additives coated on the surface of it. Due to the smooth surface of the metal foil, the active materials can easily detach from the current collector. In addition, the relatively poor chemical stability of these metal foils also results in increased internal impedanceReference Hu and Sun5 and passivation of active materials,Reference Wang, Luo, Wu, He, Zhao, Wang, Jiang and Fan6 because metal foils are susceptible to the corrosion of some electrodes and active materials. Carbon black, carbon nanotubes (CNTs) and graphite are always used as conductive additives, which account for about 10–20 wt% of the whole slurry, to improve the conductivity of the whole electrode. Nevertheless, they have no or little contribution to the capacity of the electrodes. Some side effects are also caused by the polymer binders such as polyvinylidene fluoride (PVDF), polytetrafluoroethylene, which are electrochemically inactive or even insulating. So the existence of these binders further hinders the transport of lithium-ion (Li-ions) and electrons within the active materials and compromises the overall energy density of the electrodes.
Compared with the metal foil-based electrodes, flexible and binder-free electrodes have their unique advantages in terms of portability, energy density and cycling stability over the conventional electrodes. Most of the flexible electrodes replace the traditional binders and heavy current collectors with flexible and light substrates or in-bedded current collectors. Up to now, there are mainly two approaches to make a combination of the active materials and the flexible substrates. The first one is to utilize the conductive and flexible matrix as embedded in current collector or substrates with active materials firmly entrapped on them through coating, spraying, deposing or other novel methods. The second method is adopting a nonconductive substrate with excellent mechanical properties such as polydimethylsiloxane (PDMS), plastic and cotton as substrates. Despite these novel approaches, there are still several challenges for flexible and portable energy-storage devices.
(1) Among the traditional active materials used as anodes or cathodes for LIBs and ECs, most of them are intrinsically inflexible materials such as transition metal oxides, sulfur, silicon and so on. To further apply these active materials in flexible electrodes, a mechanically robust substrate, conductive or nonconductive, must be adopted in the whole system.
(2) Liquid electrolytes are still the dominant electrolytes used in the whole flexible systems due to their excellent electrical conductivity. However, apart from the possible safety concerns like electrolyte leakage and toxicity, liquid electrolytes also limit the design and assembly of the cells, thus hindering their practical applications. Solid-state electrolytes with excellent mechanical strength and robustness hold great potential in solving the above mentioned shortages, but the development of this kind of electrolytes is still at an early stage and cannot meet the demand for flexible electronic devices.
(3) The mass loading of the active materials on the flexible materials is always compromised to achieve a high level of flexibility and mechanical strength, which is of practical importance in their applications in the portable device market.
The review is aim to summarize the recent progress in flexible LIBs and ECs, with a focus on flexible active materials and substrates, and discuss the possible challenges and prospects in the near future.
II. CNT BASED FLEXIBLE ELECTRODES
CNTs can be envisioned as rolled up graphene sheets and used as Li-ion storage materials, which can be attributed to the diffusion of Li-ion on the surface and between the layers of CNTs. In recent years, as a successfully commercialized product with high electrical conductivity, high flexibility, high Young's modulus and exceptional surface properties, CNTs have attracted enormous attention and are widely used as active materials in flexible LIBs and supercapacitors as high performance anode materials. It has been reported in the literature that it is possible to charge single-wall carbon nanotubes (SWCNTs) up to 1000 mA h/g for the first cycle,Reference Landi, Ganter, Cress, DiLeo and Raffaelle7,Reference Kim, Kojima, Muramatsu, Umemoto, Watanabe, Yoshida, Sato, Ikeda, Hayashi and Endo8 which results from effective diffusion of Li-ions on the surface and/or inside individual nanotubes.Reference Garau, Frontera, Quiñonero, Costa, Ballester and Deyà9 However, the anode exhibits an irreversible capacity during the initial cycle, which may be caused by the defects in the CNTs and the formation solid-electrolyte-interface (SEI).Reference Gwon, Hong, Kim, Seo, Jeon and Kang10 In terms of electrochemical performance, multi-wall CNTs (MWCNT) exhibit better reversible charge and discharge storage capacity than SWCNTs and double-wall CNTs.Reference Chew, Ng, Wang, Novák, Krumeich, Chou, Chen and Liu11 Apart from different types of CNTs, it is well documented that the preparation methods,Reference Gwon, Hong, Kim, Seo, Jeon and Kang10 the electrolytes,Reference Landi, Ganter, Schauerman, Cress and Raffaelle12 the surface propertiesReference Tasis, Tagmatarchis, Bianco and Prato13 and elemental doping of CNTReference Mukhopadhyay, Hoshino, Kawasaki, Okino, Hsu and Touhara14 also greatly influence the capacity of CNT as Li-ion storage materials. For example, Lee's groupReference Lee, Yabuuchi, Gallant, Chen, Kim, Hammond and Shao-Horn15 reported that the redox of oxygen-containing functional groups on the surface of CNT such as MWNT–COOH and MWNT–NH2 with Li-ions is responsible for the large gravimetric capacitances of MWNT electrodes in organic electrolytes.
Another advantage of CNT is that it can eliminate the use of conventional Cu or Al-based current collectors, conductive additives and binders and serve as flexible and porous current collectors. Therefore, various forms of CNT-based flexible materials such as yarns, films and fibers have been reported as feasible materials for broad applications.Reference Zhang, Feng, Zhai, Jiang, Li and Fan16 Meanwhile, some novel methods such as spraying,Reference Geng, Kim, So, Lee, Chang and Lee17 dip-coating,Reference Jo, Jung, Lee and Jo18 vacuum filtrationReference Hellstrom, Lee and Bao19 have also been explored to fabricate such flexible and high-performance electrodes with CNT as active materials or current collectors.
Among the recently developed methods, vacuum filtration has been demonstrated to be the most commonly used approach to fabricate CNT and active materials composite film with interconnected porous channels for fast ion and electron transport. For example, MnO2/CNT/conductive polymer (CP) film with good cycling and rate stability was synthesized by Hou et al.Reference Hou, Cheng, Hobson and Liu20 Acid treatment was used to add functional groups such as carboxylic groups or hydroxyl groups on the surface of CNTs, since the polar functional groups are able to effectively adsorb the Mn2+ ions in the solution, ensuring intimate contact between CNT and MnO2, as shown in Fig. 1. The film with a high mass loading of MnO2 (60%) delivers specific capacitance value as high as 200 F/g, which demonstrates a high utilization of the active materials with assistance of the poly(3,4-ethylenedioxythiophene)-poly(styrenesulfonic acid) (PEDOT-PSS) and the functional groups of the CNTs.

FIG. 1. (a) A schematic illustration of MnO2/CNTs/PEDOT-PSS composites. (b) Cyclic voltammograms (50 mV/s) and (c) galvanostatic charge/discharge curve (at current density of 5 mA/cm) of MnO2 film (black), MnO2/PEDOT-PSS composite (red), and MnO2/CNT/PEDOT-PSS composite (blue). Reprinted with permission from Ref. Reference Hou, Cheng, Hobson and Liu20, Copyright 2010 American Chemical Society.
Nevertheless, directly mixing the active materials and CNT via simple dispersion and filtration may yield a weak interaction between the conductive nets and active materials, resulting in a relatively poor electrochemical performance of the flexible electrodes. To address the problem, a two-step in situ method was applied by Jia et al. to synthesize LiMn2O4 based CNT films.Reference Jia, Yan, Chen, Wang, Zhang, Guo, Wei and Lu21 Layers of MnO2 were firstly generated on the surface of CNT by redox reactions between the CNTs and KMnO4. Then a hydrothermal reaction further converted the MnO2 into LiMn2O4 particles. Subsequent vacuum filtration of the LiMn2O4/CNT composites generated a self-standing and binder-free film. At a current density of 550 mA h/g, the composite material delivers a discharge capacity of 50 mA h/g. To further improve the interaction between the active materials and the conductive scaffolds, a novel strategy was reported by Cheng et al.Reference Cheng, Wang, Xin, Kim, Nie, Li, Yang and Huang22 They mixed TiO2 and CNT in water in the existence of sodium lauryl benzenesulfate (SDBS) to form a black suspension, as shown in Fig. 2. Then the black suspension was filtered by a porous membrane to form a film. A hydrothermal reaction in NaOH aqueous solution and a heating treatment were used to improve the adhesion and interaction between active materials and the conductive scaffolds by conformal coating TiO2 nanorods on the surface of CNTs. The results show that a charge capacity of 129 mA h/g is maintained after 1000 cycles at 10 C.

FIG. 2. (a) Schematic of preparing a free-standing and binder-free CNT–TiO2 film by conformal coating of TiO2 on a 3-D CNT scaffold. (b) The rate capability of the conformal CNT–TiO2 film and CNT–TiO2 mixed electrodes. (c) Cycling performance of the conformal CNT–TiO2 film electrode at a high current rate of 10 C. Reprinted with permission from Ref. Reference Cheng, Wang, Xin, Kim, Nie, Li, Yang and Huang22, Copyright 2014 Royal Society of Chemistry.
However, the disadvantages of the acid treatment and surfactants lie in the reduction of the conductivity of the CNT, which is caused by the sluggish kinetics of the functional groups and surfactants on the surface of CNTs.Reference Zhou, Li and Cheng23 Depositing silicon coatings onto CNT has been proven by Fu et al. as an effective way to utilize the remarkable properties of both CNT and silicon.Reference Fu, Yildiz, Bhanushali, Wang, Stano, Xue, Zhang and Bradford24 CNT forests were grown on a quartz substrate with SiH4 gas used as the silicon source for the uniform deposition of silicon layers. The aligned CNT provides sufficient inter-tube space for the uniform deposition of silicon coatings and accommodates the silicon's volume expansion during cycling. The cycling test shows that the charge capacity is 1494 mA h/g after 45 cycles with a capacity retention of over 94%.
CP solution can also be used as optimal “conductive surfactants” to replace the conventional surfactants or acid treatment for the preparation of free-standing and binder CNT film. Chen et al. used TiO2 nanoparticles, PEDOT:PSS solution and superlong CNTs to make a hydrogel precursor (see Fig. 3).Reference Chen, To, Wang, Lu, Liu, Chortos, Pan, Wei, Cui and Bao25 PEDOT:PSS was used as both conductive additives and surfactants since it contains both hydrophobic part and hydrophilic part. A flat film was also formed by vacuum filtration. The CNT/CP film functions as an interpenetrating network which facilitates electrode kinetics and improves strain tolerance.

FIG. 3. (a) Schematic of the aqueous solution process to fabricate flexible electrodes. (b) Photograph of the precursor solution. (c) Photograph of a composite hydrogel. (d) Photograph of an example of a flexible TiO2–PEDOT:PSS–CNT film. Reprinted with permission from Ref. Reference Chen, To, Wang, Lu, Liu, Chortos, Pan, Wei, Cui and Bao25, Copyright 2014 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
CNTs can also been spun into continuous fibers with desirable diameters and lengths through wet spinning processesReference Dalton, Collins, Muñoz, Razal, Ebron, Ferraris, Coleman, Kim and Baughman26 and chemical vapor deposition (CVD).Reference Li, Kinloch and Windle27 The advantages of these CNT-based fiber such as low density and high electrical conductivity make it attractive to be integrated with various active materials to achieve flexible and high performance electrodes.
In this respect, Lin et al. reported a CNT/silicon composite fiber anode for flexible wire-shaped LIBs.Reference Lin, Weng, Ren, Qiu, Zhang, Chen, Chen, Deng, Wang and Peng28 Aligned MWCNT arrays were synthesized by CVD followed by deposition of Si through electron beam evaporation to form a composite film. The film was further twisted into fibers and used as working electrodes with lithium wire as counter electrode. The MWCNT/Si composite fiber exhibits a specific capacity of 1042 mA h/g at 3.0 A/g after 10 cycles at 2.0 A/g and 10 cycles at 3.0 A/g. The good electrochemical performance of the CNT/Si composite fiber can be attributed to the core-sheath structure, which effectively accommodate silicon's volume changes.
III. GRAPHENE BASED FLEXIBLE ELECTRODES
The unique nanostructure of graphene with two-dimensional (2-D) carbon layers has drawn tremendous attention because it possesses numerous appealing properties such as high surface area, excellent conductivity, low weight, excellent chemical stability and high mechanical flexibility and strength.Reference Novoselov and Geim29–Reference Wang, Kou, Choi, Yang, Nie, Li, Saraf, Hu, Zhang and Graff31 Since the breakthrough made in the experimental discovery of individual graphene in 2004,Reference Novoselov, Geim, Morozov, Jiang, Zhang, Dubonos, Grigorieva and Firsov32 tremendous attempts have been made to produce individual graphene nanosheets such as CVD,Reference Kim, Zhao, Jang, Lee, Kim, Kim, Ahn, Kim, Choi and Hong33–Reference Mattevi, Kim and Chhowalla35 chemical modificationReference Stankovich, Dikin, Piner, Kohlhaas, Kleinhammes, Jia, Wu, Nguyen and Ruoff36 and chemical exfoliation.Reference Hernandez, Nicolosi, Lotya, Blighe, Sun, De, McGovern, Holland, Byrne and Gun'Ko37
Similarly, graphene can be used in both LIBs and ECs as Li-ion host materials. The electrochemical capacitance of graphene can be divided into two categories: electrochemical double-layer capacitors and pseudocapacitors. The former capacitance can be achieved through an adsorption and intercalation mechanism.Reference Raccichini, Varzi, Passerini and Scrosati38 The pseudocapacitance results from some electroactive species such as residual oxygen-containing and hydrogen-containing functional groups on the surface of graphene after various chemical modification of graphene.Reference Wang, Li, Too and Wallace39 However, it is of extraordinary challenge to directly assemble individual 2-D graphene sheet into controllable graphene-based nanoreactor or nanosystem due to its hydrophobicity, aggregation and restacking.Reference Pandolfo and Hollenkamp40 Therefore, much effort has been devoted to resolve this drawback.
As one type of graphene sheets assembled microcosmic carbon materials, graphene films are the most commonly used active materials or current collectors in flexible LIBs and ECs. In this respect, Li et al. did some pioneering works in the preparation of macroscopic scale graphene papers.Reference Li, Muller, Gilje, Kaner and Wallace41 They reported that stable aqueous colloids of chemically converted graphene (CCG) can be obtained without the need for surfactant or stabilizers. Uniform graphene films can be formed on a membrane filter by vacuum filtration of the CCG dispersions. Later, it has been demonstrated in the same group that the mechanical robustness and strength of as-synthesized CCG film can be enhanced by moderate thermal annealing.Reference Chen, Müller, Gilmore, Wallace and Li42 To further fulfil its potential in LIBs and ECs, vacuum filtration of CCG and polyaniline nanofibers (PANI-NFs) was reported by Wu et al. to form a composite film with PANI-NFs sandwiched between CCG layers.Reference Wu, Xu, Yao, Liu and Shi43 The flexible supercapacitor devices based on the film show electrochemical capacitance of 210 F/g at a discharge rate of 0.3 A/g. Yang et al. also demonstrated a liquid-mediated method by which multilayered graphene sheets can remain largely separated due to the intrinsic corrugation and colloidal interaction between as-synthesized CCG sheets.Reference Yang, Zhu, Qiu and Li44 Apart from the filtration-based methods, a facile method has also been reported by Shen et al. to fabricate large graphene dioxide (GO) sheets film by direct evaporation of GO suspension under mild heating. After graphitization at a high temperature of 2000 °C for 1 h under argon flow, the graphene film shows a graphite-like structure, excellent electromagnetic interface and in-plane thermal conductivity.Reference Shen, Zhai and Zheng45
At the same time, great efforts have been made to further improve the strength and conductivity of graphene-based flexible film, fiber or other forms of materials by enhancing the interlayer interactions between graphene sheets.Reference Gao, Liu, Zu, Peng, Zhou, Han and Zhang46 Multifunctional polymers such as multi-amino polyallylamine, have been applied to covalently crosslink graphene sheets due to the reaction between amine groups and the functional groups of the GO, but severe aggregation of GO sheets cannot be avoided to some extent.Reference Wang, Mynar, Yoshida, Lee, Lee, Okuro, Kinbara and Aida47 Later, a controllable crosslinking method was reported by Tian et al. using polydopamine as cross-linking agents, which have the capacity to adhere to various substrates.Reference Tian, Cao, Wang, Yang and Feng48 When the pH value of the system is above 8.5, the mussel-inspired polymers are able to react with amine groups.Reference LaVoie, Ostaszewski, Weihofen, Schlossmacher and Selkoe49 By tuning the pH of the solution, a controllable crosslink reaction can be realized.
However, the reversible capacity of the graphene film is still hindered by aggregation and restacking of individual graphene sheets, which inhibit the infiltration of electrolytes and the diffusion of Li-ion in both LIBs and ECs to some extent. Recently, interconnected and porous graphene materials have aroused great interest because it has been demonstrated that porous graphene structures with large surface area and interconnected nanopores are helpful in enhancing the accessibility of electrolyte and improve capacitance characteristics.Reference Chen, Ren, Gao, Liu, Pei and Cheng50–Reference Zhao, Hayner, Kung and Kung52 Chen et al. reported three-dimensional graphene foam (GF) synthesized by template-directed CVD.Reference Chen, Ren, Gao, Liu, Pei and Cheng53 Carbon was introduced into a nickel foam by decomposing CH4 at 1000 °C on the surface of the nickel foam. The interconnected and flexible network of graphene with large surface area provides fast transport channel for Li-ion and electrons. Based on this concept, the free-standing and porous GF network was fabricated and used as current collector for lithium–sulfur batteries (Li–S) by Xi et al.Reference Xi, Kidambi, Chen, Gao, Peng, Ducati, Hofmann and Kumar54 After infiltrating the sulfur solution into GF by a drop casting method, the binder-free and flexible Li–S electrodes were prepared and shows excellent rate and cycling ability with a Coulombic efficiency of 94.6% after 400 cycles at a high current density.
As mentioned previously, the porous structure of GF is necessary in improving the electrochemical performance of the GF. To further utilize the porous structure, GF with uniform and controllable pore size distribution was reported by Huang et al.Reference Huang, Qian, Yang, Zhang, Li, Yu and Zhao55 They designed a hydrophobic interaction driven hard templating approach to prepare GF with controllable pore size (30–120 nm) and high pore volumes (around 4.3 cm3/g). Silica spheres were chosen for the preparation of GF followed by etching with HF. Due to the hydrophobic and porous surface nature of the GF, metal oxide nanoparticles can be easily entrapped on the surface of the walls of GF. A typical NGF with a uniform particle size of 27.7 nm is able to deliver a large reversible capacity of about 750 mA h/g at 300 mA/g.
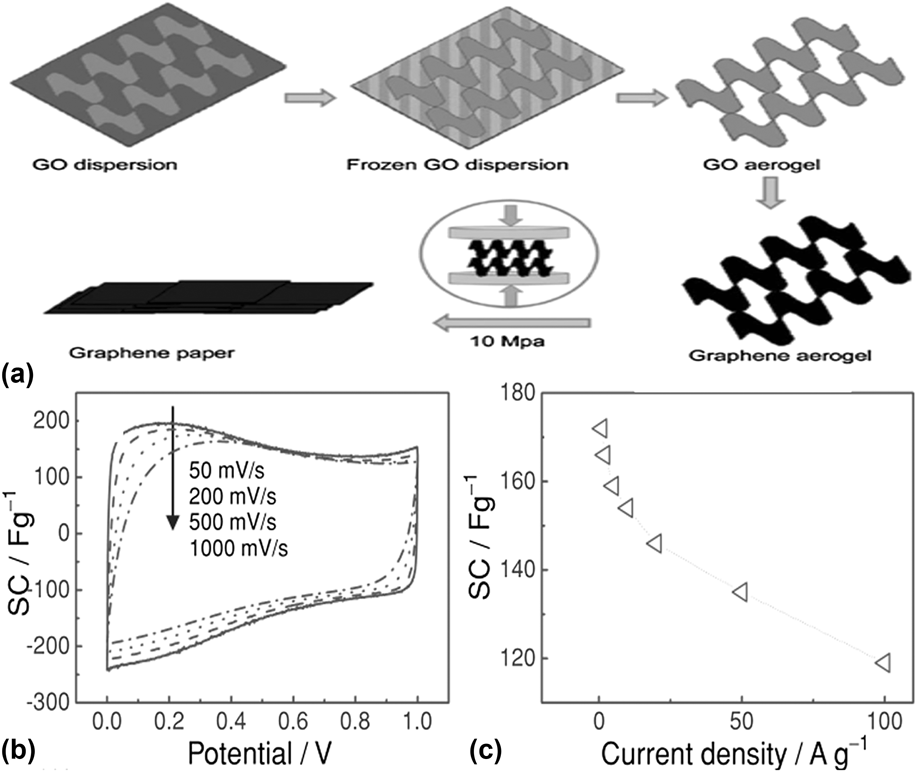
The methods discussed above require a high temperature or complex multi-step processes like CVD or etching. As shown in Fig. 4, a novel and simple strategy was reported by Liu et al. to make graphene paper with 3-D porous network by mechanically pressing a graphene aerogel, which was synthesized by freeze-drying a GO aqueous dispersion followed by thermal reduction.Reference Liu, Song, Xue and Zhang56 The folded structure of the graphene sheets with fewer layers can provide more active sites for hosting Li-ion. The electrochemical capacity is up to 172 F/g and 110 F/g at a charge/discharge rate of 1 A/g and 100 A/g, respectively, which is higher than that of graphene paper fabricated by the conventional flow-directed assembly methods.Reference Yu, Roes, Davies and Chen57

FIG. 4. (a) Illustration of the formation process of graphene paper. (b) CV curves of the graphene paper as a supercapacitor electrode at different scan rates. (c) Specific capacitance of the graphene paper as a function of charge/discharge rate. Reprinted with permission from Ref. Reference Liu, Song, Xue and Zhang56, Copyright 2012 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
When integrated with other active materials such as V2O5,Reference Gwon, Kim, Lee, Seo, Park, Lee, Ahn and Kang58 MnO2,Reference Yu, Park, Davies, Higgins, Chen and Xiao59 TiO2,Reference Deng, Kim, Lee and Cho60 silicon,Reference Wang, Li, Zhang, Luo, Jin, Liang, Dayeh, Picraux and Zhi61 sulfurReference Huang, Sun, Li, Chen and Wang62 and other active materials to form a flexible electrode, GFs and films exhibit their unique properties including extremely high specific surface area, excellent electronic conductivity and chemical stability. Moreover, the main advantage of the 2-D layer structure of graphene lies in the formation of mechanically robust matrix, the stronger adhesion between the active materials and graphene, the uniform distribution of the active materials and the elimination of the metal current collectors, conductive additives and binders.
Magnetite (Fe3O4) is regarded as a very appealing active material for LIBs due to its high theoretical capacity (926 mA h/g) and environmental benignity.Reference Zhang, Wu, Hu, Guo and Wan63 A combination of graphene film with Fe3O4 has been reported by Wang et al. to prepare a free-standing and flexible Fe3O4/graphene hybrid film.Reference Wang, Xu, Sun, Gao and Lin64 Graphene sheets functions as a 3-D conductive matrix with Fe3O4 homogeneously distributed and firmly entrapped in its porous and rough surface. Fe2O3 was used as precursor and mixed with GO to form a film via filtration. Then the film was heated at 600 °C in argon flow for 1 h for chemical conversion to fabricate Fe3O4/graphene film. The as synthesized film graphene exhibits a high specific capacity of 1555, 940 and 660 mA h/g at 100, 200 and 500 mA/g. Similarly, filtration was also used to prepare Co3O4/graphene hybrid film.Reference Wang, Xu, Sun, Liu, Gao and Lin65 Based on the total electrode weight, the hybrid film delivers a high specific capacity of 1400 and 1200 mA h/g at 100 and 200 mA/g, respectively.
Apart from the graphene films, ultrathin graphite foam (UGF) loaded with lithium iron phosphate (LFP) was used as a cathode for LIB by Ji et al.Reference Ji, Zhang, Pettes, Li, Chen, Shi, Piner and Ruoff66 LFP was mixed with carbon black and PVDF at a weight ratio of 7:2:1 in N-methyl-2-pyrrolidone to form slurry, which was drop-cast on the UGF to produce a cathode. A higher rate capability and specific capacity can be achieved (23% higher than that of the Al/LFP cathode and 170% higher than that of the Ni-foam/LFP cathode) simultaneously, owing to the conductive and 3-D interconnected graphitic structure.
In addition to the progresses achieved in various graphene papers and foams, one dimensional (1-D) graphene-based fibers are of practical importance in constructing high-strength, portable and high energy density devices. Various methods such as CVD,Reference Li, Zhao, Wang, Yang, Wei, Kang, Wu and Zhu67 electrophoretic assembly methodReference Jang, Carretero-González, Choi, Kim, Kozlov, Kim, Kang, Baek, Kim and Park68 and facile solution self-assembly strategy,Reference Tian, Xu, Li, Zhu, Shi and Lin69 have been made in the fabrication of graphene fibers.
Wet spinning has been demonstrated as a breakthrough to fabricate graphene fibers in recent years.Reference Xu and Gao70,Reference Xu, Sun, Zhao and Gao71 Various coagulation baths with different solvents such as methyl acetate, ethyl acetate and CaCl2 have been used to improve the mechanical stiffness and morphology of the graphene fibers.Reference Zhao, Jiang, Hu, Dong, Xue, Meng, Zheng, Chen and Qu72 Aboutalebi et al. reported a multifunctional graphene yarns by taking advantage of the intrinsic self-assembly behavior of ultra large graphene oxide liquid dispersions.Reference Aboutalebi, Jalili, Esrafilzadeh, Salari, Gholamvand, Aminorroaya Yamini, Konstantinov, Shepherd, Chen and Moulton73 Ultra large reduced GO (RGO) sheets with above 50 μm large sheet in size are demonstrated of great importance in achieving both high conductivity and electrochemical capacitance because of its uninterrupted surface and less grain boundaries (Fig. 5). A similar experiment conducted by Xiang et al. confirmed that larger building blocks usually result in carbon graphene-based fibers with better mechanical properties.Reference Xiang, Young, Wang, Yan, Hwang, Cerioti, Lin, Kono, Pasquali and Tour74 In their experiment, ethyl acetate was selected as the coagulation solvent for the experiment followed by a heat treatment at 1000 °C. The continuous fibers yield a modulus of 47 GPa, which was much higher than conventional pure graphene-based fibers.

FIG. 5. (a and b) Cross section view of RGO fiber. (c) Cyclic voltammograms of heat-treated graphene fiber yarns produced in an acetone bath (both free-standing and deposited on charge collectors), alkaline bath (NaOH), and ionic cross-linking using a divalent cation bath (CaCl2) in 1 M H2SO4 at 10 mV/s. (d) Calculated specific capacitance of RGO fiber yarns fabricated in an acetone bath at various scan rates. Reprinted with permission from Ref. Reference Aboutalebi, Jalili, Esrafilzadeh, Salari, Gholamvand, Aminorroaya Yamini, Konstantinov, Shepherd, Chen and Moulton73, Copyright 2014 American Chemistry Society.
In addition to the conventional methods, a novel facile one-step dimensionally confined hydrothermal strategy was reported by Qu's group to prepare a neat graphene fiber from GO suspensions.Reference Dong, Jiang, Cheng, Zhao, Shi, Jiang and Qu75 A glass pipeline was used as the reactor and aqueous GO suspension was injected into the glass pipeline, followed by low-temperature heat treatment at 230 °C for 2 h. A strong and low-weight graphene fiber well matching the pipe geometry was produced. Later, to extend the applications of as-synthesized fibers, MnO2-modified graphene fiber has been fabricated by direct deposition of MnO2 onto the surface of graphene fiber in the same group.Reference Chen, Meng, Hu, Zhao, Shao, Chen and Qu76
IV. GRAPHENE AND CNT HYBRID ELECTRODES
Recently, combining 1-D CNTs and 2-D graphene sheets to fabricate a composite film or fiber has attracted great attention since this method is efficient in utilizing the both merits of CNT and graphene and alleviating the aggregation and restacking of individual graphene sheets during the preparation process.Reference Hu, Li, Wang, Li and Sun77 CNTs serve as both conductive additives and structural pillows, which not only facilitate the infiltration of the electrolyte, but also improve the conductivity and electrochemical performance of the whole electrodes simultaneously. Simply by sonicating a mixture of GO dispersion and CNTs powder (0.05 wt%, respectively), a very stable and black suspension can be formed without visible sediment and the addition of any surfactants, indicating that GO acts as an amphiphilic molecule to realize the homogenous dispersion of CNTs.Reference Qiu, Yang, Gou, Yang, Ma, Wallace and Li78
Compared with the conventional CNT-based and graphene-based fiber, graphene/CNT composite fibers for energy-storage devices show their unique mechanical and electrochemical properties. In the experiment reported by Sun et al., GO solution was added in the CNT array to form composite films, which were scrolled into fibers at the rotary rate of 2000 rpm.Reference Sun, You, Deng, Chen, Yang, Ren and Peng79 CNTs function as effective bridges to improve the charge transport and decrease the contact resistances. The tensile strength of the graphene/CNT composite fiber is 630 MPa. When used as supercapacitors, the fiber delivers a specific capacitances of up to 31.50 F/g. Similar coaxial wet-spun yarn supercapacitor (YSC) based on graphene and CNT was also reported by Gao' group.Reference Kou, Huang, Zheng, Han, Zhao, Gopalsamy, Sun and Gao80 They demonstrated for the first time a coaxial wet-spinning assembly strategy to prepare core-sheath fibers. GO and CNT dispersions were first mixed with weight ratio of 1/1 followed by transferring to an injection syringe connected with an inner core of spinneret. Sodium carboxymethyl cellulose was transferred to the outer channel of spinneret to form a novel core-sheath structure (Fig. 6). The resulting compact two-ply YSCs show high capacitance and energy density values up to 269 mF/cm2 (239 F/cm3) and 5.91 mW h/cm2 (5.26 mW h/cm3) using liquid electrolyte.

FIG. 6. (a) Scanning electron microscope (SEM) images of cross-sectional view of a two-ply YSC. (b) SEM image of a two-ply YSC knot. (c) Galvanostatic charge and discharge curves of single, two and three YSCs connected in series. (d) CV curves of single, two and three YSCs connected in parallel, and the insets show the corresponding schematics (at a scan rate of 10 mV/s). Reprinted with permission from Ref. Reference Kou, Huang, Zheng, Han, Zhao, Gopalsamy, Sun and Gao80, Copyright 2014, Nature Publishing Group.
In conclusion, an improved electrochemical performance of these hybrid electrodes was achieved due to the synergistic effect of both CNT and graphene. Graphene works as the main substrates with CNT as multifunctional pillows, which further enhance the conductivity, electrochemical capacity and mechanical properties of the whole system.
V. OTHER CONDUCTIVE MATRIX BASED FLEXIBLE ELECTRODES
Despite the progress made in flexible electrodes by using CNT and graphene as both active materials and current collectors, other forms of carbon materials and some novel mental-based current collectors are also widely explored to achieve flexible electrodes with excellent properties.
It has been demonstrated in previous discussion that the surface condition of the active materials greatly influence their electrochemical properties. Carbon cloth-based solid-state supercapacitor was prepared by Wang et al. via the modification of carbon fiber.Reference Wang, Wang, Lu, Ling, Yu, Zhai, Tong and Li81 They reported an effective chemical oxidation and reduction method to increase surface area of the carbon materials. HNO3, H2SO4 and KMnO4 were used as oxidizing reagents to modify the surface of carbon cloth and form a rough surface. Figure 7 shows a core–shell structure at the edge of each carbon fiber. Then the modified carbon fibers were reduced by hydrazine and ammonia. PVA and H2SO4 were used to prepare the solid-state electrolyte. The symmetric solid state supercapacitor device exhibits a capacitance of 15.3 mF/cm2 with a rate retention of 76% at 10 mV/s achieved at 1000 mV/s.

FIG. 7. (a) Low-magnification TEM image collected at the edge of the oxidized carbon fiber. (b) High-resolution TEM image showing the exfoliated carbon fibers. Reprinted with permission from Ref. Reference Wang, Wang, Lu, Ling, Yu, Zhai, Tong and Li81, Copyright 2014 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Carbon cloth can also be used as conductive substrates with active materials deposited on the surface of it. MoS2 nanoflakes were grown on the surface of carbon fiber and used as flexible LIB anode.Reference Yu, Zhu, Zhang, Chen, Li, Gao, Yang and Ouyang82 One-step hydrothermal synthesis method was adopted to prepare hierarchical MoS2 flakes with MoO3, thioacetamide, and urea as precursors. When used as a flexible LIB anode, the flexible 3-D MoS2 has a high reversible capacity of 3.0–3.5 mA h/cm2 at a current density of 0.15 mA/cm2 and outstanding rate stability. Similarly, Liu et al. reported a flexible anode with ZnCo2O4 nanowire uniformly deposited on carbon fiber (see Fig. 8).Reference Liu, Zhang, Wang, Chen, Chen, Zhou and Shen83 A two-step process was needed to synthesize hierarchical ZnCo2O4 nanowire. The binder-free anodes deliver a high reversible capacity of 1300–1400 mA h/g and excellent cycling ability even after 160 cycles with a capacity of 1200 mA h/g.

FIG. 8. (a) Schematic illustration of the synthesis of flexible 3-D ZnCo2O4 nanowire arrays/carbon cloth. (b) Capacity versus cycle number plot at different charging rates. (c) Long-term cycling of the ZnCo2O4 nanowire arrays/carbon cloth electrode with a reversible capacity value of 1200 mA h/g1 after 160 cycles with Coulombic efficiency of 99%. Reprinted with permission from Ref. Reference Yu, Zhu, Zhang, Chen, Li, Gao, Yang and Ouyang82, Copyright 2014 Royal Society of Chemistry.
In 2005, a new type of carbon nanofiber with novel bamboo-like structure was reported and shows excellent mechanical flexibility, foldability and electrochemical performances.Reference Sun, Sills, Hu, Seh, Xiao, Xu, Luo, Jin, Xin, Li, Zhang, Zhou, Cai, Huang and Cui84 Polyacrylonitrile and tetraethyl orthosilicate in dimethylformamide were used to make the nanofibers by electrospinning. After heating the precursors at 1200 °C in a H2/Ar atmosphere, ultrafine SiO2 clusters aggregate into much larger SiO2 particles, which were etched by HF aqueous solution to generate interior holes. In the fiber, an even distribution of the holes not only enhances its mechanical stability, but also reduces ion diffusion resistance. The specific power and energy densities of the nanofibers are 61.3 kW/kg and 2.37 Wh/kg, respectively.
As 1-D conductive substrates, metal wires such as Ni and Ti fibers, are typically explored for efficient electrode materials in a variety of energy-storage devices due to their high electrical conductivity. Wu et al. demonstrated a ZnCo2O4 nanorod based flexible supercapacitor with Ni wire as current collector and flexible substrate.Reference Wu, Lou, Yang and Shen85 A simple and rapid one-step hydrothermal process was taken in the preparation of the electrode. The result shows that, 97.9% and 92% of the initial capacitance are maintained after 800 times cycles at varied current and 3500 charge/discharge cycles, respectively.
Nickel foam can not only be used as templates to synthesize GF with interconnected network, but also can serve as highly conductive and 3-D scaffold for flexible batteries. The unique structure of nickel foam play an important role in reducing the diffusion resistance of electrolytes and enhancing ion transportation by providing very smooth electron pathways.Reference Yang, Xu and Li86 Up to now, nickel foam has been mostly reported in many literature and used as flexible current collector. Liu et al. reported that nickel foam can be used to offer flexible room for the accommodation of volume expansion of Si.Reference Liu, Huang, Fan, Zhang, Sun, Gao, Yang and Zhong87 Si nanowires (SiNWs) were dispersed in water with SDBS as surfactant. Then Ni foam was immersed in the SiNWs solution for 30 min followed by pressing at 4 MPa CR2032 coin was assembled to test the electrode and it achieves an initial capacity of as high as 2486 mA h/g with a reversible capacity of 1500 mA h/g retained even after 50 cycles of charging and discharging.
Due to the high conductivity and 3-D interconnected structure of nickel foam, it is one of the most optimal substrates for electrodeposition methods with various nanomaterials deposited on the surface of it and used as active materials. Yang et al. reported a supercapacitors by electrodepositing Ni(OH)2 on nickel foam with.Reference Yang, Xu and Li86 The electrode was tested in the potential range of 0.05–0.45 V with a maximum specific capacitance as high as 3152 F/g in 3% KOH solution at a charge/discharge current density of 4 A/g. The enhancement of the specific capacitance is mainly attributed to the even distribution of Ni(OH)2 on the surface of conductive Ni substrate and the intimate contact between the active materials and the conductive substrate. Similar electrodepositing method was also applied by Chen's group, as shown in Fig. 9.Reference Zhao, Hu, Zhang, Zhang, Hu and Chen88 They reported a high-performance cathode for Li–S batteries with nickel foam as a flexible and binder-free current collector. Electrodepostion method was applied to prepare reactive sulfur nanodots with average size of 2 nm, which are uniformly distributed on the surface of Ni foam. Sulfur content could be controlled from 0.21 to 4.79 mg/cm2 simply by controlling the deposition time. The electrode delivers a high initial discharge capacity (1458 mA h/g at 0.1 C), high rate capability (521 mA h/g at 10 C) and long cycling stability (528 mA h/g after 1400 cycles at 5 C).

FIG. 9. (a) A schematic diagram of preparing sulfur nanodots on Ni foam with an electrodeposition method. (b) Discharge and charge profiles at 0.1 C. (c) Cycling performance of S nanodots cathodes with different S content and bulk S cathode (without the addition of Li2S8). (d) Rate performance of S nanodots cathodes. Reprinted with permission from Ref. Reference Zhao, Hu, Zhang, Zhang, Hu and Chen88, Copyright 2015 American Chemical Society.
VI. NONCONDUCTIVE SUBSTRATES
Compared with the conductive substrates, other nonconductive substrates with inherent advantages in flexibility, mechanical robustness and porosity, such as commonly used separators,Reference Li, Yang, Hu, Wang, Li, Cai, Li and Sun89 PDMS,Reference Won, Kim, Yang, Jeong and Moon90 poly(m-phenylene isophthalamide),Reference Jiang, Zhang, Song, Ma, Li, Lee, Han and Liu91 textilesReference Jost, Perez, McDonough, Presser, Heon, Dion and Gogotsi92 and other polymers, have also been widely applied in the flexible electrodes to obtain high performance flexible and bendable electrodes.
Commercially used separator with inherent flexibility is an indispensable part of the whole LIB system to prevent short circuit, so the energy density will not be sacrificed when they are used as flexible substrates. Celgard 3500 have been explored by Li et al. to fabricate a binder-free and current collector-free anode.Reference Li, Yang, Hu, Wang, Li, Cai, Li and Sun89 Entangled CNTs were trapped on the surface of the separator after filtration and served as active materials. Based on this concept, Zhou et al. designed a novel sandwich structure with pure sulfur trapped in two membranes.Reference Zhou, Pei, Li, Wang, Wang, Huang, Yin, Li and Cheng93 One graphene membrane was used as a current collector (GCC) with pure sulfur coated on it as active materials. To further improve the mechanical robustness and flexibility of the electrode, a commercial polymer separator (G-separator) was coated with another graphene layer and used as both separator and current collector as shown in Fig. 10. The excellent flexibility of the electrodes should be attributed to the mechanical robustness of both polymer separator and graphene film.

FIG. 10. (a) Photographs of an as-prepared large-area GCC strip (width: 6 cm) with good flexibility. (b) 3-D reconstructed G-separator. (c) Cycling stability of the Li–S batteries with GCC/S+G-separator at 1.5 A/g1 for 300 cycles. Reprinted with permission from Ref. Reference Zhou, Pei, Li, Wang, Wang, Huang, Yin, Li and Cheng93, Copyright 2014 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
From the above discussion, we know that graphene films and foams are widely used as flexible current collectors for LIBs and ECs. However, the mechanical properties of the graphene-based materials limit their further application. In addition to the separators mentioned before, PDMS is used to further improve the flexibility of graphene films and foams via different methods. Zhou et al. developed a flexible Li–S battery electrode by using GF as a current collector and host for large sulfur loading via a simple slurry infiltration.Reference Zhou, Li, Ma, Wang, Shi, Koratkar, Ren, Li and Cheng94 The PDMS coated on the surface of GF makes the interconnected network sufficiently robust, ensuring the flexibility of the cathode. The high sulfur-loaded electrodes retain a high rate performance of 450 mA h/g under a large current density of 6 A/g and preserve stable cycling performance with 0.07% capacity decay per cycle over 1000 cycles, as shown in Fig. 11.

FIG. 11. (a) Schematic of the procedure for fabricating PDMS/GF and S-PDMS/GF electrodes. (b) Rate performance of the S-PDMS/GF electrodes with different sulfur loadings. (c) Cycling performance an Coulombic efficiency of the S-PDMS/GF electrode with a 10.1 mg/cm2 sulfur loading at 1500 mA h/g for 1000 cycles. Reprinted with permission from Ref. Reference Zhou, Li, Ma, Wang, Shi, Koratkar, Ren, Li and Cheng94, Copyright 2015 Elsevier Ltd.
In addition to the hosts and substrates for graphene-based materials, PDMS is also able to be used as transparent and stretchable substrate for CNT. A highly aligned carbon nanotube sheets with excellent optical transmittance and mechanical stretchability was developed by Chen et al. as all-solid supercapacitors.Reference Chen, Peng, Durstock and Dai95 Vertically aligned CNT was synthesized by CVD with ethylene as the carbon source followed by directly drawing the horizontally aligned CNT sheet onto a PDMS substrate. The transparent supercapacitor exhibits a specific capacitance of 7.3 F/g and can be stretched up to 30% strain without any obvious structural deformation and capacity decay even over hundreds of stretching cycles. To further utilize the unique properties of PDMS, a porous, stretchable, and conductive PDMS-based electrodes embedded with carbon CNTs was reported by Lee et al. for LIBs.Reference Lee, Yoo, Park, Kim, Kang and Jung96 In their experiment, polymethylmethacrylate was added and then removed to generate well-controlled pore networks. It has been demonstrated that the porous nature of the nanocomposites plays an important role in controlling the infiltration and accessibility of the electrolyte. As a result, an improvement of 670% in capacity of the porous nanocomposite has been achieved compared to that of a nonporous nanocomposite.
Despite the advanced performance for flexible LIBs and ECs, most electrode materials are only synthesizable in certain nanostructures or carbon-based templates, which may bring about high cost and manufacturing complexity. Textile and cellulose are naturally abundant resource and have potential applications in future wearable electronics due to their mechanical strength and low cost. Hu et al. reported wearable power devices based on natural textiles using an extremely simple “dipping and drying” process.Reference Hu, Pasta, Mantia, Cui, Jeong, Deshazer, Choi, Han and Cui97 SDBS was used as surfactant to form well-dispersed SWNTs. Then a textile was dipped into the suspension and subsequently dried in an oven. The textile with a conductivity of 125 S/cm and sheet resistance less than 1 Ω/sq was produced. They also demonstrated that the loading of pseudocapacitor materials MnO2 could be achieved by electrodeposition.
Similarly, natural mica was used as sacrificial substrates for flexible all-solid LIBs. Koo et al. reported a new universal transfer approach with LiCoO2, lithium phosphorus oxynitride and a lithium metal as cathode, solid electrolyte and anode, respectively.Reference Koo, Park, Lee, Suh, Jeon, Choi, Kang and Lee98 Packaged in PDMS, the thin-film LIB is capable of a maximum 4.2 V charging voltage and 106 μA h/cm2 capacity. Meanwhile, the battery could operate a flexible organic LED system when integrated into a single platform, which indicate the high performance for flexible LIBs, as shown in Fig. 12.

FIG. 12. (a) Photograph of a bendable LIB turning on a blue LED in bent condition. (b) Robustness tests of a flexible LIB on a bending stage machine. (c) Cross-sectional SEM image of a thin-film LIB. (d) Coulombic efficiencies of a flexible LIB bent to R c = 3.1 mm and a mica LIB. Reprinted with permission from Ref. Reference Koo, Park, Lee, Suh, Jeon, Choi, Kang and Lee98, Copyright 2012 American Chemical Society.
From the above discussion, it can be concluded that, due to the excellent mechanical properties and chemical stability of PDMS, it is widely used in various energy-storage devices and serves as stretchable and transparent substrates for LIBs and ECs. Meanwhile, some natural materials have gradually attracted increasing attention as emerging substrates for flexible LIBs and ECs.
VII. ELECTROLYTES
As a key component of LIBs or ECs, the selection of suitable electrolytes has a great influence on both the performance and design of the flexible LIBs and ECs. Conventionally, the electrolyte serves as both Li-ion diffusion media and insulator to prevent direct contact between the two electrodes in the system. In flexible electrodes, the electrolytes should possess more properties such as high ionic conductivity, good mechanical robustness, low risk of electrolyte leakage and low toxicity.
Electrolytes are commonly divided into liquid electrolytes and solid electrolytes. In terms of ionic conductivity and the physical contact between the electrodes and the electrolytes, liquid electrolytes exhibit their advantages over that of solid electrolytes. So they are mostly used in the existing LIBs or ECs. However, after repeated deformation or bending, there is a higher risk of the leakage of liquid electrolytes and the related safety concerns such as flammability, toxicity and explosion. As a result, elaborate packaging and assembly of these cells are highly required to prevent the possible short-circuit and potential dangers.
Solid-state electrolytes, especially the shape-comfortable solid-state electrolytes discussed in this essay, are considered to be safer and more reliable than liquid organic electrolytes because of their chemical stability, low metallic corrosion and minimal risk of electrolyte leakage. However, the practical applications of the conventional solid-state electrolytes were hindered by its low ionic conductivity, brittleness and narrow electrochemical window stability.Reference Barpanda, Chotard, Delacourt, Reynaud, Filinchuk, Armand, Deschamps and Tarascon99 The breakthrough was made in plastic crystal electrolytes (PCEs) and gel-polymer electrolytes (GPEs). For example, Ha et al.Reference Ha, Kil, Kwon, Kim, Lee and Lee100 demonstrated a facile approach based on integration of a UV (ultraviolet)-curable semi-interpenetrating polymer network (semi-IPN) matrix with a PCE to achieve improved mechanical bendability and electrochemical performance. In addition, no decomposition of any components in the plastic crystal composite electrolyte (PCCE) takes place below 5.0 V versus Li+/Li, indicating stable electrochemical window of the PCCEs. Incorporating liquid electrolytes into polymer matrix also forms solid-state electrolytes with improved mechanical properties. Recently, a facile and scalable approach was reported by Kil et al. in the same group to prepare highly ion-conductive and bendable polymer electrolytes.Reference Kil, Choi, Ha, Xu, Rogers, Kim, Lee, Kim, Cho and Lee101 The GPE is composed of a UV-cured polymer matrix, high-boiling point liquid electrolyte with alumina (Al2O3) nanoparticles used as a functional filler to control the rheological properties of the electrolyte mixture.
VIII. CONCLUSION AND FUTURE PROSPECTS
This review summarized and discussed the recent progresses made in flexible electrodes for LIBs and ECs, with a focus on different flexible active materials and substrates. As lightweight, thin and flexible matrix, carbon-based materials such as CNT, graphene and carbon fibers are still the dominant materials that are used as active materials for flexible electrodes in LIBs and ECs. However, the weaknesses of these materials lie in its low reversible capacity and low Coulombic efficiency, which hinders its practical applications and commercialization. When used as flexible substrates and embedded-in current collectors, the carbon-based materials show excellent mechanical strength and chemical stability. In addition, novel metal foams/fibers, natural materials and some nonconductive polymers are also widely adopted as mechanically robust substrates and exhibit great potential in the further utilization.
To achieve scalable and low-cost production of flexible energy-storage devices such as LIBs and ECs in the near future, improvements can be made in the reduction of the costs of flexible substrates, the selection of suitable electrolytes and simplification of the fabrication process. Some safety concerns should also be addressed by optimizing the preparation parameters, electrolytes along with the packaging materials. Meanwhile, improving the mechanical strength of both the electrodes and electrolyte is necessary to further realize the applications of these flexible LIBs and ECs in roll-up displays and wearable devices.
At the same time, as novel electrochemical nanomaterials in the area of energy-storage devices, comprehensive measurement standards and assessment criterions are needed for both the electrochemical and mechanical performance of these flexible electrodes. For example, the criterions should involve some necessary factors such as the intensity and types of deformation, bending time and radii, stretchability and mechanical wearability to accurately and systematically characterize the flexible properties of the electrodes.
Finally, various methods need to be explored to search for novel materials with both excellent mechanical and electrochemical properties. Meanwhile, increasing the mass loading of these active materials on the flexible electrodes is urgently required to further improve its energy density of the electrodes without compromising the volumetric energy density.
As the era of smart electronics and next-generation flexible energy storage and conversion systems has come, flexible electronics market will continue to grow. Despite the challenges discussed above in this essay, the unique advantages and properties of the flexible electrodes will be further explored to meet increasing demands in flexible electronics market.
ACKNOWLEDGMENT
This work Supported by Natural Science Foundation of Shanxi Province (Grant No: 2015021062).