Article contents
Processing of biphasic calcium phosphate ceramics for culturing of bone marrow stem cells
Published online by Cambridge University Press: 04 April 2017
Abstract
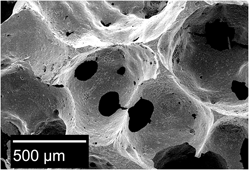
Ceramic scaffolds are being developed to control both proliferation and differentiation of hematopoietic stem cells into desired cell products in bioreactors. These scaffolds mimic important aspects of the microenvironment or “niche” inside bone marrow. In particular, hematopoietic stem cell fate is thought to be effected by the architecture of trabecular bone and the presence of calcium. Here we report the effects of ceramics processing on the phase distribution and microstructure of biphasic ceramics used to culture hematopoietic stem cells. Processing of biphasic ceramics by powder mixing resulted in tetracalcium phosphate on the sintered surface. This correlated with observed surface deposits, weight gain, and the release of calcium ions in saline over 28 days. In contrast, impregnation of partially sintered hydroxyapatite with calcium nitrate resulted in calcium carbonate on the sintered surface. Impregnation correlated with the release of calcium ions into the saline, surface pitting, and weight loss.
- Type
- Invited Articles
- Information
- Journal of Materials Research , Volume 32 , Issue 17: Focus Issue: Achieving Superior Ceramics and Coating Properties through Innovative Processing , 14 September 2017 , pp. 3260 - 3270
- Copyright
- Copyright © Materials Research Society 2017
Footnotes
Contributing Editor: Eugene Medvedovski
References
REFERENCES
- 3
- Cited by



