Article contents
Pressure-induced metallic phase transition and elastic properties of indium phosphide III-V semiconductor
Published online by Cambridge University Press: 14 March 2012
Abstract
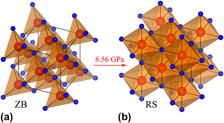
In this work, we find that the pressure-induced phase transition of InP from III-V semiconductor phase having zincblende (ZB) crystal structure to metallic phase having rocksalt (RS) structure occurs at a pressure of 8.56 GPa accompanied by an 18% volume collapse. It is found that the nearest In and P atoms bonded as covalent bond. Crystal space of ZB is just occupied by In-P tetrahedrons partly with many interstices, but that of RS is fulfilled by close-packed octahedrons entirely. With pressures, broadened energy band of antibonding state and the reduced density of states (DOS) of bonding state cause the weakening of tetrahedral In-P covalent bonds. And then, ZB is destroyed and rebuilt to RS structure. Some In-5s, In-5p, P-3p and a few P-3s move to unoccupied high energy level, across Fermi level, and migrate from valence band to conduction band, and then generate metallic properties. Furthermore, changes of covalent bond would cause evident variation of elastic properties on the {100} and {110} planes.
- Type
- Articles
- Information
- Copyright
- Copyright © Materials Research Society 2012
References
REFERENCES
- 5
- Cited by


