Crossref Citations
This article has been cited by the following publications. This list is generated based on data provided by
Crossref.
Heinhold, R.
Williams, G. T.
Cooil, S. P.
Evans, D. A.
and
Allen, M. W.
2013.
Influence of polarity and hydroxyl termination on the band bending at ZnO surfaces.
Physical Review B,
Vol. 88,
Issue. 23,
Lord, Alex M.
Maffeis, Thierry G.
Allen, Martin W.
Morgan, David
Davies, Philip R.
Jones, Daniel R.
Evans, Jonathan E.
Smith, Nathan A.
and
Wilks, Steve P.
2014.
Surface state modulation through wet chemical treatment as a route to controlling the electrical properties of ZnO nanowire arrays investigated with XPS.
Applied Surface Science,
Vol. 320,
Issue. ,
p.
664.
Ching, Kwong-Lung
Li, Guijun
Ho, Yeuk-Lung
and
Kwok, Hoi-Sing
2016.
The role of polarity and surface energy in the growth mechanism of ZnO from nanorods to nanotubes.
CrystEngComm,
Vol. 18,
Issue. 5,
p.
779.
Lord, Alex M
Evans, Jonathan E
Barnett, Chris J
Allen, Martin W
Barron, Andrew R
and
Wilks, Steve P
2017.
Surface sensitivity of four-probe STM resistivity measurements of bulk ZnO correlated to XPS.
Journal of Physics: Condensed Matter,
Vol. 29,
Issue. 38,
p.
384001.
Chernysheva, Ekaterina
Srour, Waked
Philippe, Bertrand
Baris, Bulent
Chenot, Stéphane
Duarte, Roberto Felix
Gorgoi, Mihaela
Cruguel, Hervé
Rensmo, Håkan
Montigaud, Hervé
Jupille, Jacques
Cabailh, Gregory
Grachev, Sergey
and
Lazzari, Rémi
2018.
Band alignment at Ag/ZnO(0001) interfaces: A combined soft and hard x-ray photoemission study.
Physical Review B,
Vol. 97,
Issue. 23,
Murdoch, B.J.
Raeber, T.J.
Zhao, Z.C.
Barlow, A.J.
McKenzie, D.R.
McCulloch, D.G.
and
Partridge, J.G.
2019.
Light-gated amorphous carbon memristors with indium-free transparent electrodes.
Carbon,
Vol. 152,
Issue. ,
p.
59.
Swallow, J. E. N.
Varley, J. B.
Jones, L. A. H.
Gibbon, J. T.
Piper, L. F. J.
Dhanak, V. R.
and
Veal, T. D.
2019.
Transition from electron accumulation to depletion at β-Ga2O3 surfaces: The role of hydrogen and the charge neutrality level.
APL Materials,
Vol. 7,
Issue. 2,
Lopis, Antony Dasint
Choudhari, K.S.
Sai, Ranajit
Kanakikodi, Kempanna S.
Maradur, Sanjeev P.
Shivashankar, S.A.
and
Kulkarni, Suresh D.
2022.
Laddered type-1 heterojunction: Harvesting full-solar-spectrum in scavenger free photocatalysis.
Solar Energy,
Vol. 240,
Issue. ,
p.
57.
Lucero Manzano, Andrea M.
Fuhr, Javier D.
Cantero, Esteban D.
Famá, Marcelo
Sánchez, Esteban A.
Esaulov, Vladimir E.
and
Grizzi, Oscar
2022.
Hydroxylation of the Zn terminated ZnO(0 0 0 1) surface under vacuum conditions.
Applied Surface Science,
Vol. 572,
Issue. ,
p.
151271.
Xiao, Yun
Wang, Haibin
Awai, Fumiyasu
Shibayama, Naoyuki
Kubo, Takaya
and
Segawa, Hiroshi
2022.
Emission Spectroscopy Investigation of the Enhancement of Carrier Collection Efficiency in AgBiS2-Nanocrystal/ZnO-Nanowire Heterojunction Solar Cells.
ACS Applied Materials & Interfaces,
Vol. 14,
Issue. 5,
p.
6994.
Picasso, Carolina
Salinas, Yolanda
Brüggemann, Oliver
Scharber, Markus Clark
Sariciftci, Niyazi Serdar
Cardozo, Olavo D. F.
Rodrigues, Eriverton S.
Silva, Marcelo S.
Stingl, Andreas
and
Farias, Patricia M. A.
2022.
Lanthanide (Eu, Tb, La)-Doped ZnO Nanoparticles Synthesized Using Whey as an Eco-Friendly Chelating Agent.
Nanomaterials,
Vol. 12,
Issue. 13,
p.
2265.
Sitkov, Nikita
Ryabko, Andrey
Kolobov, Alexey
Maximov, Alexsandr
Moshnikov, Vyacheslav
Pshenichnyuk, Stanislav
Komolov, Alexei
Aleshin, Andrey
and
Zimina, Tatiana
2023.
Impedimetric Biosensor Coated with Zinc Oxide Nanorods Synthesized by a Modification of the Hydrothermal Method for Antibody Detection.
Chemosensors,
Vol. 11,
Issue. 1,
p.
66.
Ryabko, A. A.
Nalimova, S. S.
Permyakov, N. V.
Bobkov, A. A.
Maksimov, A. I.
Kondratev, V. M.
Kotlyar, K. P.
Ovezov, M. K.
Komolov, A. S.
Lazneva, E. F.
Moshnikov, V. A.
and
Aleshin, A. N.
2024.
Architectonics of Zinc Oxide Nanorod Coatings for Adsorption Gas Sensors.
Technical Physics,
Vol. 69,
Issue. 7,
p.
2103.
Alaizeri, ZabnAllah M.
Alhadlaq, Hisham A.
Aldawood, Saad
and
Abduh, Naaser A. Y.
2024.
Green synthesis of ZnO-TiO2/RGO nanocomposites usingSenna surattensisextract: a novel approach for enhanced anticancer efficacy and biocompatibility.
RSC Advances,
Vol. 14,
Issue. 24,
p.
16685.
Baker, M.A.
Bacon, S.R.
Sweeney, S.J.
Hinder, S.J.
Bushell, A.
Nunney, T.S.
and
White, R.G.
2024.
Femtosecond laser ablation (fs-LA) XPS – A novel XPS depth profiling technique for thin films, coatings and multi-layered structures.
Applied Surface Science,
Vol. 654,
Issue. ,
p.
159405.
Vorochta, Michael
Mansfeldova, Vera
Chakraborty, Samiran
and
Kavan, Ladislav
2024.
Work function and electrochemistry of ZnO (wurtzite) single crystals (F-1:L07).
Ceramics International,
Lopis, Antony Dasint
Choudhari, K.S.
Sai, Ranajit
Sudarshana
Kanakikodi, Kempanna S.
Maradur, Sanjeev P
and
Kulkarni, Suresh D.
2024.
Co2+-laddered heterojunction a next-generation solar-photocatalyst: Unusually improved activity for the decomposition of pharmaceuticals, dyes, and microplastics.
Materials Research Bulletin,
Vol. 176,
Issue. ,
p.
112836.
Kimura, Kento
Marangon, Vittorio
Fukuda, Taiga
Suzuki, Mana
Soontornnon, Nantapat
Tominaga, Yoichi
and
Hassoun, Jusef
2024.
TEMPO-oxidized cellulose nanofiber hydrogel electrolyte for rechargeable Zn-ion batteries.
Chemical Communications,
Vol. 60,
Issue. 93,
p.
13698.
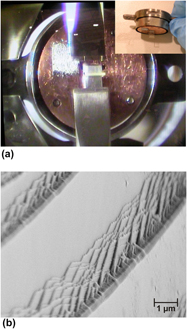
 ) faces of ultrahigh vacuum cleaved hydrothermally grown ZnO single crystals. The cleaved polar surfaces showed a characteristic polarity effect in that the intensity of emission from the lowest binding energy O 2p related valence band states was significantly stronger on the Zn-polar face, even when the cleaved surfaces were imperfect with irregular nonatomically flat features. A residual submonolayer hydroxyl termination of approximately 0.5 ML was observed on both the Zn-polar and O-polar surfaces immediately after cleaving. The near-surface downward band bending on the O-polar face was removed by the cleaving process leaving almost flat bands, while on the cleaved Zn-polar face, emission from states above the valence band edge was observed.
) faces of ultrahigh vacuum cleaved hydrothermally grown ZnO single crystals. The cleaved polar surfaces showed a characteristic polarity effect in that the intensity of emission from the lowest binding energy O 2p related valence band states was significantly stronger on the Zn-polar face, even when the cleaved surfaces were imperfect with irregular nonatomically flat features. A residual submonolayer hydroxyl termination of approximately 0.5 ML was observed on both the Zn-polar and O-polar surfaces immediately after cleaving. The near-surface downward band bending on the O-polar face was removed by the cleaving process leaving almost flat bands, while on the cleaved Zn-polar face, emission from states above the valence band edge was observed.