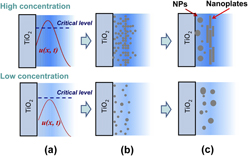
Crossref Citations
This article has been cited by the following publications. This list is generated based on data provided by
Crossref.
Zhan, Li
He, Jialun
Wang, Weiping
Zheng, Xuanli
Cao, Yiyan
Yin, Jun
Kong, Lijing
Zou, Qiang
Bhutto, Waseem Ahmed
Chen, Xiaohong
Li, Shuping
Wu, Zhiming
and
Kang, Junyong
2016.
Optimized design of multi-shell ZnO/TiO2/ZnSe nanowires decorated with Ag nanoparticles for photocatalytic applications.
RSC Advances,
Vol. 6,
Issue. 75,
p.
71800.
Piwoński, Ireneusz
Spilarewicz-Stanek, Kaja
Kisielewska, Aneta
Kądzioła, Kinga
Cichomski, Michał
and
Ginter, Joanna
2016.
Examination of Ostwald ripening in the photocatalytic growth of silver nanoparticles on titanium dioxide coatings.
Applied Surface Science,
Vol. 373,
Issue. ,
p.
38.
Li, Shuai
Li, Dawei
Zhang, Qing-Yu
and
Tang, Xin
2016.
Surface enhanced Raman scattering substrate with high-density hotspots fabricated by depositing Ag film on TiO2-catalyzed Ag nanoparticles.
Journal of Alloys and Compounds,
Vol. 689,
Issue. ,
p.
439.
Fu, Xin
Pan, Lujun
Li, Shuai
Wang, Qiao
Qin, Jun
and
Huang, Yingying
2016.
Controlled preparation of Ag nanoparticle films by a modified photocatalytic method on TiO2 films with Ag seeds for surface-enhanced Raman scattering.
Applied Surface Science,
Vol. 363,
Issue. ,
p.
412.
Xu, W.
Wang, S. Q.
Zhang, Q. Y.
Ma, C. Y.
Li, X. N.
Wang, Q.
and
Wen, D. H.
2017.
Abnormal Oxidation of Ag Films and Its Application to Fabrication of Photocatalytic Films with a-TiO2/h-Ag2O Heterostructure.
The Journal of Physical Chemistry C,
Vol. 121,
Issue. 18,
p.
9901.
Li, HongWei
Zhu, Hekai
Wang, Meng
Min, Xin
Fang, Minghao
Huang, Zhaohui
Liu, Yan’gai
and
Wu, Xiaowen
2017.
A new Ag/Bi7Ta3O18 plasmonic photocatalyst with a visible-light-driven photocatalytic activity.
Journal of Materials Research,
Vol. 32,
Issue. 19,
p.
3650.
Xu, W.
Wang, S.Q.
Zhang, Q.Y.
Ma, C.Y.
Wang, Q.
Wen, D.H.
and
Li, X.N.
2019.
Hierarchically structured AgO films with nano-porosity for photocatalyst and all solid-state thin film battery.
Journal of Alloys and Compounds,
Vol. 802,
Issue. ,
p.
210.
Zammouri, Lobna
Aboulaich, Abdelhay
Capoen, Bruno
Bouazaoui, Mohamed
Sarakha, Mohamed
Stitou, Mostafa
and
Mahiou, Rachid
2019.
Synthesis of YAG:Ce/ZnO core/shell nanoparticles with enhanced UV-visible and visible light photocatalytic activity and application for the antibiotic removal from aqueous media.
Journal of Materials Research,
Vol. 34,
Issue. 08,
p.
1318.
Wiercigroch, Ewelina
Kisielewska, Aneta
Blat, Aneta
Wislocka, Adrianna
Piwoński, Ireneusz
and
Malek, Kamilla
2019.
Photocatalytical decoration of thin titania coatings with silver nanostructures provides a robust and reproducible SERS signal.
Journal of Raman Spectroscopy,
Vol. 50,
Issue. 11,
p.
1649.
Hoffmann, Manuel
Wackerow, Stefan
Abdolvand, Amin
and
Zolotovskaya, Svetlana A.
2021.
High performance SERS platforms via parametric optimization of the laser-assisted photodeposition of silver and gold nanoparticles.
Optical Materials Express,
Vol. 11,
Issue. 9,
p.
3079.
Sun, Ning
Huang, Bo
Lv, Zhenyin
Ran, Na
Gan, Yuan
and
Zhang, Jie
2024.
UV-Catalyzed TiO2–Based Optofluidic SERS Chip for Three Online Strategies: Fabrication, Detection, and Self-Cleaning.
Analytical Chemistry,
Vol. 96,
Issue. 22,
p.
9104.
Kong, Fanyi
Ji, Chenhua
Zhao, Gaolei
Zhang, Lei
Hao, Zheng
Wang, Hu
Dai, Jianxun
Huang, Huolin
Pan, Lujun
and
Li, Dawei
2024.
Controlled Fabrication of Wafer-Scale, Flexible Ag-TiO2 Nanoparticle–Film Hybrid Surface-Enhanced Raman Scattering Substrates for Sub-Micrometer Plastics Detection.
Nanomaterials,
Vol. 14,
Issue. 19,
p.
1597.
Li, Shuai
and
Zhang, Qing-Yu
2025.
Photocatalytic growth of Ag nanoparticles on TiO2 films: growth behavior and kinetics study by UV–Vis-NIR extinction spectroscopy and scanning electron microscopy.
Journal of Nanoparticle Research,
Vol. 27,
Issue. 3,