No CrossRef data available.
Article contents
Osteogenic differentiation of mesenchymal stem cells on hybrid coatings sterilized by different processes
Published online by Cambridge University Press: 14 October 2019
Abstract
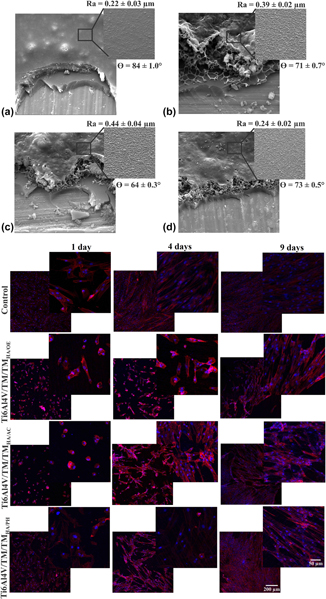
The objective of the present work was to evaluate the behavior of osteogenesis of mesenchymal stem cells (MSCs) on a double-layer, protective, and bioactive hybrid coating sterilized by 3 different processes: steam autoclave, hydrogen peroxide plasma, and ethylene oxide. The hybrid coating was obtained from a sol consisting of the silane precursors tetraethoxysilane (TEOS) and methyltriethoxysilane (MTES), applied on a Ti6Al4V substrate. To promote bioactivity, hydroxyapatite (HA) particles were dispersed in a second coating (bioactive layer: TEOS/MTES + HA) applied on the first (TEOS/MTES). The sterilized coatings were evaluated by scanning electron microscopy, wettability, and micrometer roughness. The behavior of hydrolytic degradation was evaluated by the mass variation of the samples and the release of silicon by the technique of high-resolution atomic absorption spectrometry. All coatings presented morphological and superficial alterations after sterilization. Sterilization by ethylene oxide and hydrogen peroxide plasma intensified the hydrolytic degradation of the bioactive coating causing a greater release of silicon. The sterilized hybrid coatings did not show cytotoxicity to MSCs. Adhesion, viability, and osteogenic differentiation were favored on the sterilized coating of hydrogen peroxide plasma, which is opposite to what was observed for the ethylene oxide-sterilized coating.
- Type
- Article
- Information
- Copyright
- Copyright © Materials Research Society 2019


