Article contents
One-pot synthesis of core–shell structured Sn/carbon nanotube by chemical vapor deposition and its Li-storage properties
Published online by Cambridge University Press: 26 September 2011
Abstract
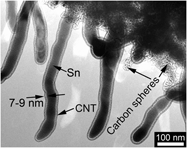
Core–shell structured Sn/carbon nanotube (CNT) was prepared by one-pot chemical vapor deposition (CVD) method in N2/C2H2 (10% C2H2) using nanosized SnO2 as the starting material. The obtained one-dimensional material is composed of a disordered carbon shell and a single-crystalline Sn nanorod core. The diameter of the Sn nanorod and the thickness of the carbon shell are around 40–50 and 4–5 nm, respectively, when the CVD reaction was carried out at 650 °C for 2 h. The core–shell structured Sn/CNT exhibits improved electrochemical performance compared with bare Sn with a diameter of around 100 nm. A reversible capacity of around 350 mAh/g can be retained after 20 cycles at 50 mA/g for Sn/CNT, while for bare Sn, the capacity drops rapidly to 100 mAh/g after the same cycles.
- Type
- Articles
- Information
- Copyright
- Copyright © Materials Research Society 2011
References
REFERENCES
- 4
- Cited by


