Published online by Cambridge University Press: 26 August 2020
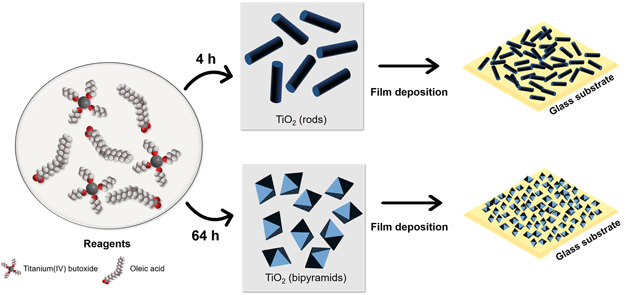
Titanium dioxide (TiO2) is a semiconductor that can be applied in different technological areas. In this work, we investigated the modifications of the electrical properties of thin films composed of TiO2 nanoparticles produced with different morphologies. The solvothermal route used for the synthesis allowed the production of nanoparticles with functionalized surfaces due to oleate groups. It was possible to modulate nanocrystals shape and size due to the detachment crystal growth mechanism, by changing the reaction time. Nanorods were obtained using 4 h of synthesis, and an increase in the reaction time to 64 h led to a bipyramidal morphology. The functionalization by the organic ligand allowed the preparation of stable colloidal solutions, which were used to prepare thin films by the dip-coating method. The films presented a homogeneous surface, an average thickness around 100 nm, and no agglomerations were observed. The electrical resistance measurements indicated a typical behavior of semiconductors, and they were dependent on the nanoparticle morphology. An exploratory test indicated that the thin films prepared using nanorod particles presented a higher electrical response compared with isotropic particles, when exposed in a liquefied petroleum gas vapor atmosphere. Therefore, the morphology of the nanoparticles is a key factor for the further application of these thin films in gas sensing. Employing an easy methodology which required simple apparatus, and by using reaction time modulation only, it was possible to prepare homogeneous thin films with a tunable electrical response.
Contributing Editor: Edson leite