Published online by Cambridge University Press: 04 July 2016
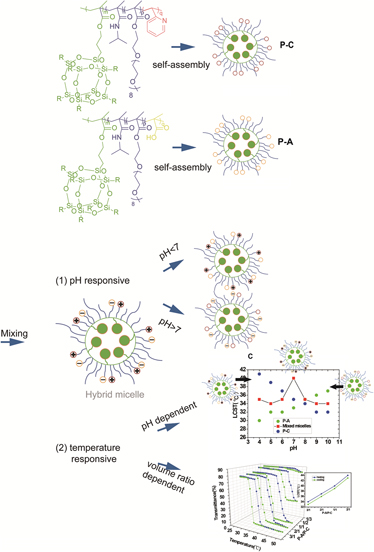
Novel mixed micelle was successfully fabricated by the synergistic self-assembly of poly(methacrylate isobutyl polyhedral oligomeric silsesquioxane (POSS)-co-N-isopropylacrylamide-co-oligo(ethylene glycol)methyl ether methacrylate-co-acrylic acid) (P(methacrylate isobutyl (MAPOSS)-co-NIPAM-co-OEGMA-co-AA)) and poly(methacrylate isobutyl POSS-co-N-isopropylacrylamide-co-oligo(ethylene glycol) methyl ether methacrylate-co-2-vinylpyridine) (P(MAPOSS-co-NIPAM-co-OEGMA-co-2VP)). Dynamic light scattering (DLS) and transmission electron microscopy characterizations demonstrate that the formation of mixed micelles is driven by electrostatic interaction. The formation of the mixed micelles was further implied by a simple fluorescence resonance energy transfer based technique. The mixed micelle possesses the biggest size at pH = 7.0, which is attributed to the strongest electrostatic interaction between the two kinds of micelles. The zeta potential under different pH was detected to further investigate the surface charges corroborating the discussions. DLS and UV-vis indicate that the lower critical solution temperature (LCST) is pH dependent. The mixed micelles reach the highest LCST at pH 7.0. The LCST of the mixed micelle can be tuned by adjusting the volume ratio of the two kinds of micelles as well. Moreover, the thermo-responsive behavior of the mixed micelle is absolutely reversible.
Contributing Editor: Tao Xie