Article contents
The microstructure of scale formed by oxynitriding of Ti and exhibiting significant apatite-forming ability
Published online by Cambridge University Press: 10 March 2016
Abstract
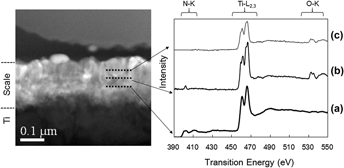
A scale formed by heat treatment of Ti in a nitrogen atmosphere containing oxygen at an extremely low partial pressure exhibited an exceptional degree of hydroxyapatite (HAp) formation in a simulated body fluid. Scanning transmission electron microscopy and electron energy loss spectroscopy indicated that the subsurface of this scale was composed of nitrogen doped rutile-type TiO2. The N-K edge energy-loss near edge structure spectrum of this layer in conjunction with the theoretical spectra of possible compounds obtained using the augmented plane wave plus local orbital band method suggested that oxygen sites were replaced by two nitrogens, resulting in an effective charge of +2. The enhanced HAp forming ability of this scale is likely related to the positively charged surface induced by the presence of N. Conversely, the subsurface scale formed by heat treatment in air, in which N is not found, leads to much slower HAp coverage, believed to be related to the lack of surface charge.
- Type
- Articles
- Information
- Copyright
- Copyright © Materials Research Society 2016
References
REFERENCES
- 5
- Cited by


