Article contents
Localized surface plasmon resonance sensing properties of photocatalytically prepared Ag/TiO2 films
Published online by Cambridge University Press: 31 January 2011
Abstract
Photocatalytic deposition of Ag nanoparticles from a mixed aqueous solution of AgNO3 and polyvinylpyrrolidone (PVP) onto TiO2 film supported on indium-tin oxide-coated glass slide (Ag/TiO2 film) was carried out by using UV light irradiation. The size and shape of the Ag nanoparticles were controlled by the addition of PVP during the photodeposition. In the optical absorption spectra of the Ag/TiO2 film, the localized surface plasmon resonance (LSPR) absorption of the Ag nanoparticles was observed. When the film was immersed in various kinds of alcohols with the refractive index at 20°C (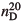 ), ranging between 1.3292 and 1.4103, the peak LSPR absorption was shifted to longer wavelengths and the peak absorbance increased with increasing
), ranging between 1.3292 and 1.4103, the peak LSPR absorption was shifted to longer wavelengths and the peak absorbance increased with increasing 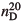 . The spectral change of the Ag/TiO2 film with larger spherical Ag nanoparticles was more prominent than that with smaller ones.
. The spectral change of the Ag/TiO2 film with larger spherical Ag nanoparticles was more prominent than that with smaller ones.
Keywords
- Type
- Articles
- Information
- Journal of Materials Research , Volume 25 , Issue 1: Photocatalysis for Energy and Environmental Sustainability , January 2010 , pp. 117 - 123
- Copyright
- Copyright © Materials Research Society 2010
References
REFERENCES
- 6
- Cited by


