Published online by Cambridge University Press: 23 January 2019
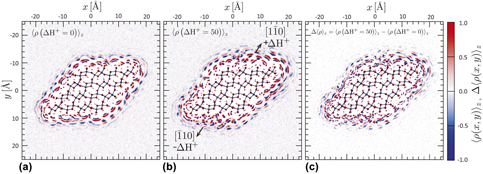
When a metal oxide surface is immersed in aqueous solution, it has the ability to bind, orient, and order interfacial water, affecting both chemical and physical interactions with the surface. Structured interfacial water thus possesses time-averaged, spatially varying polarization charge and potential that are comparable to those arising due to ion accumulation. It is well established that interfacial water structure propagates from the surface into bulk solution. Here, we show that interfacial water structure also propagates laterally, with important consequences. The constant pH molecular dynamics was used to impose a pH difference between opposite faces of a model goethite (α-FeOOH) nanoparticle and quantify water polarization charge on intervening faces. We find that the structure of water on one face is strongly affected by the structure on nearby surfaces, revealing the importance of long-range lateral hydrogen bonding networks with implications for particle aggregation, oriented attachment, and processes such as dissolution and growth.
This author was an editor of this journal during the review and decision stage. For the JMR policy on review and publication of manuscripts authored by editors, please refer to http://www.mrs.org/editor-manuscripts/.