Published online by Cambridge University Press: 24 April 2017
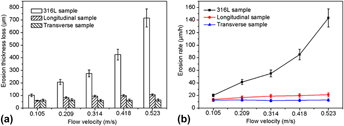
The erosion behavior of a directionally solidified Fe–B alloy containing 3.5 wt% B in the different velocities of flowing liquid zinc is investigated by X-ray diffraction and scanning electron microscopy to clarify the effect of interaction between Fe2B and the erosion products on erosion performance using a rotating-disk technique. The results indicate that the Fe–B alloy erodes at a low and steady rate in flowing liquid zinc. The microstructure of erosion layers of the directionally solidified Fe–B alloy depends on the orientation relation between the growth direction of Fe2B phase and the erosion surface. When the growth direction of Fe2B is perpendicular to the erosion surface, the Fe2B and the erosion compounds form a compact and stable combining layer that effectively inhibits the erosion of flowing liquid zinc and further improves the erosion resistance of Fe–B alloy.
Contributing Editor: Jürgen Eckert