Article contents
Interfacial enhancement of poly(ethylene terephthalate)/silica composites using graphene oxide
Published online by Cambridge University Press: 09 August 2012
Abstract
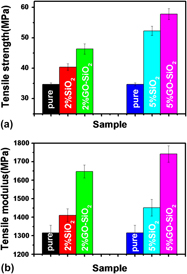
In this work, a novel core–shell structured hybrid graphene oxide-encapsulated silica (GO–SiO2) was first fabricated via an electrostatic assembly between negatively charged graphene oxide (GO) sheets and positively charged sub-micro-sized silica. Then, the new kind of hybrid filler was used for the in situ preparation of poly(ethylene terephthalate) (PET)/GO–SiO2 composites. The microstructure and mechanical properties of the prepared composites were analyzed by scanning electron microscopy, transmission electron microscopy, thermogravimetric analysis, dynamic mechanical analysis measurements, and tensile test. It was found that GO could be covalently assembled onto the subsized silica surface via its plenty of functional groups that can also provide strong interaction with the PET. As a result, a uniform dispersion of GO–SiO2 hybrids and enhanced interfacial adhesion as well as improved mechanical property have been evidenced. The new concept of using GO as a potent inorganic fillers surface modifier may broaden the use of GO and provides a new idea for the design and fabrication of advanced polymer composites.
- Type
- Articles
- Information
- Copyright
- Copyright © Materials Research Society 2012
References
REFERENCES
- 6
- Cited by


