Article contents
Influences of brush plating solutions composition and technological parameters on the quality of rolled copper foil surface coatings
Published online by Cambridge University Press: 15 December 2015
Abstract
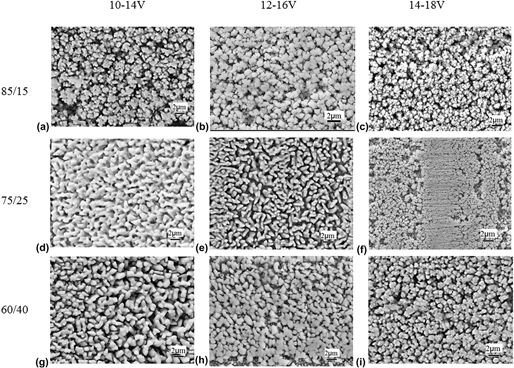
The technologies of brush plating and dealloying were used to treat the surface of rolled copper foil. Zn–Ni and Sn–Zn alloy coatings were prepared. The laws of plating solutions composition and technological parameters on coatings quality were investigated. The results show that with the decreasing of main elements mass ratio or increasing of brush time, thickness and corrosion resistance of Zn–Ni alloy coating increase. With the increasing of brush plating voltage or time, surface roughness of Sn–Zn alloy coating decreases. Turning up brush plating voltage could raise deposition rate of sub-tin and zinc ions and refine surface grains of coating. The angle of dealloying has the significant effect on the roughness of dealloyed Sn–Zn alloy coating. As the dealloying angle increases, surface roughness of dealloyed Sn–Zn alloy coating increases. The contribution of dealloying time to surface roughness of treated coating is obviously larger than that of corrosion solution concentration.
Keywords
- Type
- Articles
- Information
- Copyright
- Copyright © Materials Research Society 2015
Footnotes
Contributing Editor: Jürgen Eckert
References
REFERENCES
- 1
- Cited by


