Published online by Cambridge University Press: 19 May 2020
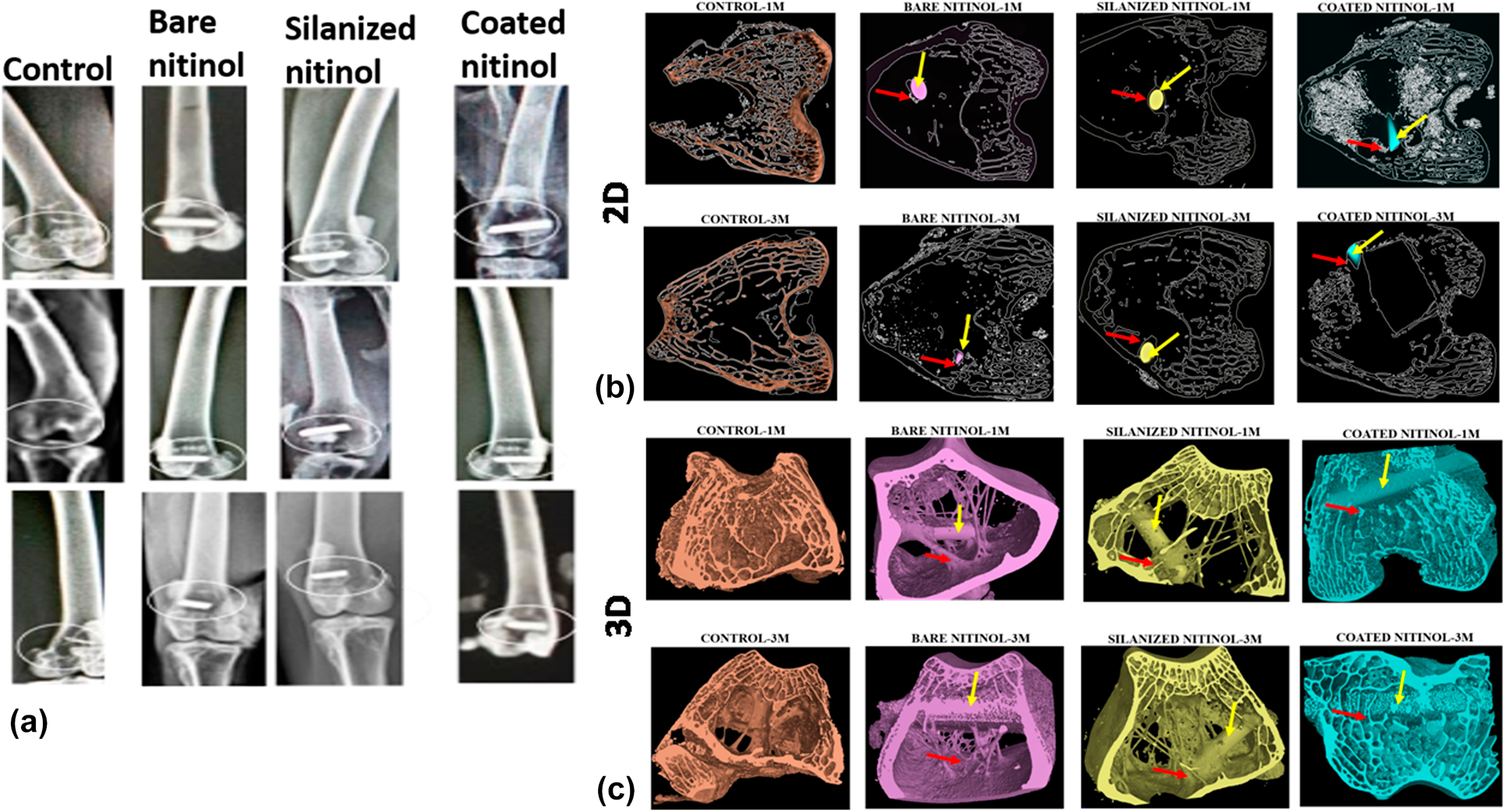
Some interesting properties such as superelasticity, shape memory effect, kink resistance, good biocompatibility, biomechanical properties, and corrosion resistance made nitinol a popular biomaterial as stent and orthopedic implants. But surface modification is needed to control nickel leaching from its surface, making safe for human body. The aim of this study was to modify the nitinol surface by the silanization technique and electrophoretically deposited hydroxyapatite coating, and to conduct a detailed in vitro and in vivo investigation. Detailed in vitro investigation involved MTT assay with the human osteoblastic cells (MG63 cell) over a period of 5 days and confocal image study. In case of in vivo study, histological study, fluorochrome labeling study, and Micro-Ct study were conducted. The overall in vitro and in vivo results indicate that silanized nitinol samples are showing slightly better level of performance, but both the surface-modified samples are suitable as the potential bio-implant for orthopedic purpose.
Present address: Department of Chemistry, Nims Institute of Engineering & Technology, Nims University Rajasthan, Jaipur-Delhi Highway (NH-11C), Nims Institute of Engineering & Technology, Jaipur-303121, Rajasthan, India.