Published online by Cambridge University Press: 21 February 2019
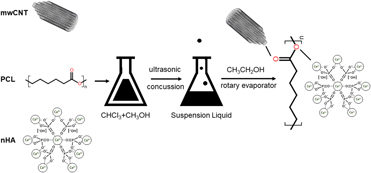
In this study, a three-phased multiwalled scaffold, composed of carbon nanotube (mwCNT), nanocrystalline hydroxyapatite (nHA), and polycaprolactone (PCL), was fabricated by the solvent evaporation technique. The structure character, mechanical properties, and degradation activity in simulated body fluid (SBF), along with osteoproductive ability in human osteosarcoma cell MG63, were investigated thoroughly. Results showed that the three phases in mwCNT/nHA/PCL composite presented excellent miscibility and stronger interfacial force when the weight content was 1/15/84 (wt%). Simultaneously, the composite had smaller porosity and slower degradation rate, and there was massive crystallized hydroxyapatite formed on the surface after being soaked in SBF. With regard to bioactivity, MG63s on this scaffolds presented good proliferation performance and differentiated into the osteogenic lineage by expressing high levels of ALP. It was concluded that mwCNTs/nHA/PCL composite scaffolds might be beneficial for bone tissue engineering at a relatively low concentration of mwCNTs and nHA.
These authors contributed equally to this work.