Article contents
Highly efficient phase transfer catalyst supported on Janus composite particles: Synthesis, characterization, and applications
Published online by Cambridge University Press: 20 June 2014
Abstract
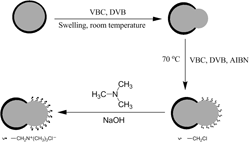
Groups of chloromethyl were randomly grafted to Janus composite particles and quaternization was carried out on the particles. The Janus material of titania–quaternary ammoniated poly(vinylbenzyl chloride–divinylbenzene) (TiO2–QApoly(VBC–DVB)) was investigated in detail. Results revealed that the anisotropic Janus material containing quaternary ammonium groups was synthesized successfully. The Janus material could be used as the phase transfer catalyst. The catalytic activity of the Janus material was confirmed by the esterification reaction of benzyl chloride and sodium acetate. In the presence of 0.5 wt.% (relative to benzyl chloride) of TiO2–QApoly(VBC–DVB), the esterification yield of benzyl acetate reached 87.6% when the molar ratio of sodium acetate anhydrous to benzyl chloride was 1.2. The catalyst exhibited a high activity and had no obvious loss of activity when recycled three times. Moreover, the Janus material was easily recovered by centrifugation and washed with ethanol and water.
- Type
- Articles
- Information
- Copyright
- Copyright © Materials Research Society 2014
References
REFERENCES
- 3
- Cited by


