Crossref Citations
This article has been cited by the following publications. This list is generated based on data provided by
Crossref.
Hallstedt, Bengt
2013.
Thermodynamic evaluation of the Al–V–C system.
Calphad,
Vol. 41,
Issue. ,
p.
156.
Jiang, Yan
Mráz, Stanislav
and
Schneider, Jochen M.
2013.
Growth of V–Al–C thin films by direct current and high power impulse magnetron sputtering from a powder metallurgical composite target.
Thin Solid Films,
Vol. 538,
Issue. ,
p.
1.
Agne, Matthias T.
and
Barsoum, Michel W.
2016.
Enthalpy of formation and thermodynamic parameters of the MAX phase V2AlC.
Journal of Alloys and Compounds,
Vol. 665,
Issue. ,
p.
218.
Wang, Zhenyu
Li, Xiaowei
Zhou, Jie
Liu, Pei
Huang, Qing
Ke, Peiling
and
Wang, Aiying
2016.
Microstructure evolution of V–Al–C coatings synthesized from a V 2 AlC compound target after vacuum annealing treatment.
Journal of Alloys and Compounds,
Vol. 661,
Issue. ,
p.
476.
Wang, Chenxu
Yang, Tengfei
Xiao, Jingren
Liu, Shaoshuai
Xue, Jianming
Huang, Qing
Zhang, Jie
Wang, Jingyang
Wang, Yugang
and
Koc, R.
2016.
Structural Transitions Induced by Ion Irradiation in V2AlC and Cr2AlC.
Journal of the American Ceramic Society,
Vol. 99,
Issue. 5,
p.
1769.
Shang, Lin
Konda Gokuldoss, Pradeep
Sandlöbes, Stefanie
to Baben, Moritz
and
Schneider, Jochen M.
2017.
Effect of Si additions on the Al2O3 grain refinement upon oxidation of Cr2AlC MAX phase.
Journal of the European Ceramic Society,
Vol. 37,
Issue. 4,
p.
1339.
Shang, Lin
to Baben, Moritz
Pradeep, Konda Gokuldoss
Sandlöbes, Stefanie
Amalraj, Marshal
Hans, Marcus
Chang, Keke
Music, Denis
Primetzhofer, Daniel
and
Schneider, Jochen M.
2017.
Phase formation of Nb2AlC investigated by combinatorial thin film synthesis and ab initio calculations.
Journal of the European Ceramic Society,
Vol. 37,
Issue. 1,
p.
35.
Cheverikin, Vladimir
Fartushna, Iuliia
and
Watson, Andy
2018.
Al-C-V Ternary Phase Diagram Evaluation.
MSI Eureka,
Vol. 73,
Issue. ,
p.
10.24005.1.0.
Shabalin, Igor L.
2020.
Ultra-High Temperature Materials III.
p.
515.
Azina, Clio
Mráz, Stanislav
Greczynski, Grzegorz
Hans, Marcus
Primetzhofer, Daniel
Schneider, Jochen M.
and
Eklund, Per
2020.
Oxidation behaviour of V2AlC MAX phase coatings.
Journal of the European Ceramic Society,
Vol. 40,
Issue. 13,
p.
4436.
Zhang, Zerong
Qian, Yuhai
Xu, Jingjun
Zuo, Jun
and
Li, Meishuan
2021.
Effect of annealing on microstructure evolution and corrosion resistance of an amorphous Cr-Al-C coating.
Corrosion Science,
Vol. 178,
Issue. ,
p.
109062.
Lee, Jinho
Kwon, Suh-Young
and
Lee, Ju Han
2022.
Harmonically mode-locked Er-doped fiber laser at 1.3 GHz using a V2AlC MAX phase nanoparticle-based saturable absorber.
Optics & Laser Technology,
Vol. 145,
Issue. ,
p.
107525.
Wei, Liangxiao
Liu, Xuyang
Gao, Youzhi
and
Hu, Ning
2022.
TMS 2022 151st Annual Meeting & Exhibition Supplemental Proceedings.
p.
1497.
Tang, Chongchong
Dürrschnabel, Michael
Jäntsch, Ute
Klimenkov, Michael
Steinbrück, Martin
Ulrich, Sven
Hans, Marcus
Schneider, Jochen M.
and
Stüber, Michael
2023.
Synthesis of V2AlC thin films by thermal annealing of nanoscale elemental multilayered precursors: Incorporation of layered Ar bubbles and impact on microstructure formation.
Applied Surface Science,
Vol. 629,
Issue. ,
p.
157340.
Yuan, Jianghuai
Wang, Zhenyu
Ma, Guanshui
Bai, Xiaojing
Li, Yong
Cheng, Xiaoying
Ke, Peiling
and
Wang, Aiying
2023.
MAX phase forming mechanism of M–Al–C (M = Ti, V, Cr) coatings: In-situ X-ray diffraction and first-principle calculations.
Journal of Materials Science & Technology,
Vol. 143,
Issue. ,
p.
140.
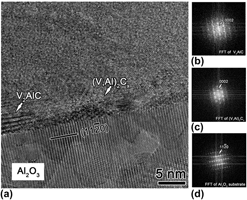
 $(11\mathop 2\limits^ - 0)$ substrates at 500 °C by direct current magnetron sputtering using a powder metallurgical composite target with 2:1:1 MAX phase stoichiometry. Transmission electron microscopy (TEM) and x-ray diffraction results suggest that a hexagonal Al-containing vanadium carbide solid solution (V,Al)2Cx was formed. The films exhibited a strong basal plane texture. The lattice parameter of the hexagonal solid solution was dependent on the annealing temperature: the c lattice parameter decreased by 3.45% after annealing for 1 h at 750 °C compared to the as-deposited film. Based on the comparison between experimental and theoretical lattice parameter data, it is reasonable to assume that this annealing-induced change in lattice parameter is a consequence of atomic ordering. Meanwhile, the formation of V2AlC MAX phase was observed at 650 °C and phase-pure V2AlC was obtained at 850 °C. TEM images support the notion that V2AlC forms by nucleation and growth.
$(11\mathop 2\limits^ - 0)$ substrates at 500 °C by direct current magnetron sputtering using a powder metallurgical composite target with 2:1:1 MAX phase stoichiometry. Transmission electron microscopy (TEM) and x-ray diffraction results suggest that a hexagonal Al-containing vanadium carbide solid solution (V,Al)2Cx was formed. The films exhibited a strong basal plane texture. The lattice parameter of the hexagonal solid solution was dependent on the annealing temperature: the c lattice parameter decreased by 3.45% after annealing for 1 h at 750 °C compared to the as-deposited film. Based on the comparison between experimental and theoretical lattice parameter data, it is reasonable to assume that this annealing-induced change in lattice parameter is a consequence of atomic ordering. Meanwhile, the formation of V2AlC MAX phase was observed at 650 °C and phase-pure V2AlC was obtained at 850 °C. TEM images support the notion that V2AlC forms by nucleation and growth.

