Published online by Cambridge University Press: 29 May 2020
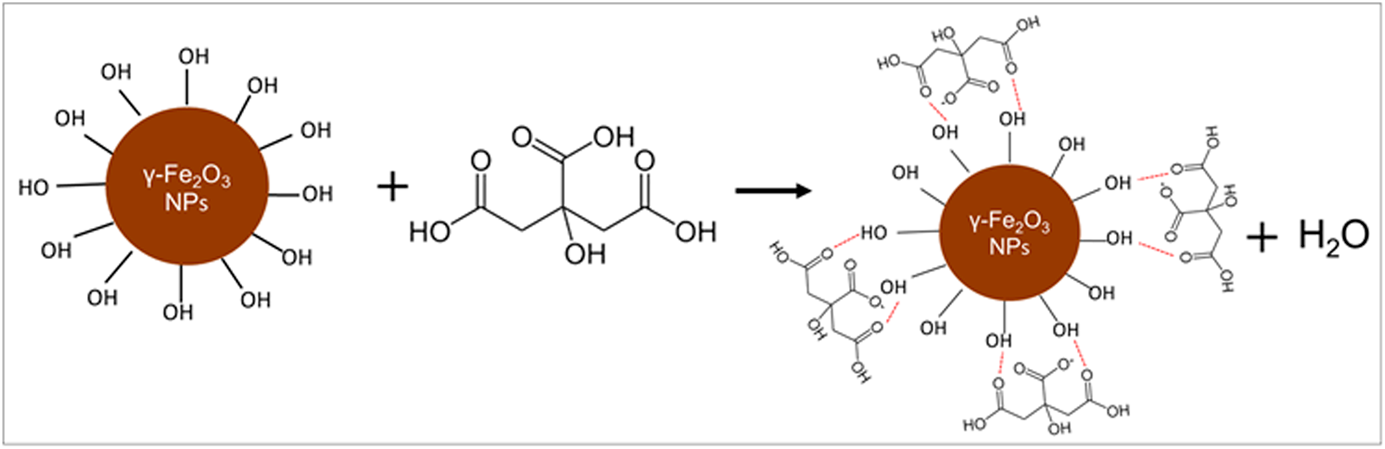
Iron oxide nanoparticles presenting colloidal stability in water were prepared through precipitation and then surface-functionalized with varying citric acid (CA) concentrations (0.10, 0.25, 0.50, and 0.70 g/mL). CA introduced functionality and minimized agglomeration. Iron oxide nanoparticles with colloidal stability in water at physiological pH were obtained after functionalization with 0.25–0.70 g/mL CA, whereas iron oxide nanoparticles without stability in water were obtained after functionalization with 0.10 g/mL CA. An electrode for glucose detection was fabricated by self-assembling colloidal-stable γ-Fe2O3 NP–CA in water on indium tin oxide (ITO) glass, followed by a glucose oxidase (GOx) and Nafion layer. The optimal functionalization of the γ-Fe2O3 NPs was obtained at a CA concentration of 0.25 g/mL. The electrochemical properties and electrocatalytic behavior of the modified electrode designated as Nafion/GOx/γ-Fe2O3 NP–0.25 CA/ITO were then evaluated. The electrode showed high sensitivity for glucose detection of 995.57 and 5.81 µA/(mM cm2) within the linear ranges of 0.1–5.0 µM and 5.0 µM–20.0 mM, respectively. The modified electrode also demonstrated a low limit of detection, good repeatability of 2.5% (n = 10), and sufficient reproducibility of 3.2% (n = 5).