Article contents
Functionalization of petroleum coke-based mesoporous carbon for synergistically enhanced capacitive performance
Published online by Cambridge University Press: 13 February 2017
Abstract
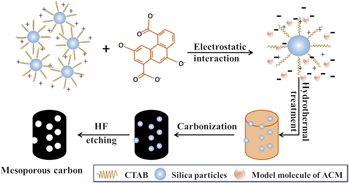
With increasing output of petroleum coke, the value-added exploitation of petroleum coke has become a tough problem. Preparing porous carbons is a traditional way to the value-added exploitation of petroleum coke. Here, we used a facile and efficient hard-templating strategy to synthesize mesoporous carbon with high surface area from petroleum coke. N2 adsorption analyses show that the BET specific area and pore volume of the carbons can reach up to 864 m2/g and 1.37 cm3/g, respectively. To utilize the abundant mesopores of the carbons, anthraquinone-modified mesoporous carbon was tested as an electrode material for supercapacitor applications. Electrochemical measurements demonstrated that the specific capacitance reached up to 366 F/g at the current density of 1 A/g, indicating a promising prospect of using this carbon in electrochemical energy-storage field. More importantly, the strategy used in this work can be easily modified to prepare other nano-carbon materials from petroleum coke.
- Type
- Articles
- Information
- Copyright
- Copyright © Materials Research Society 2017
Footnotes
Contributing Editor: Mauricio Terrones
References
REFERENCES
- 7
- Cited by



