Published online by Cambridge University Press: 11 July 2012
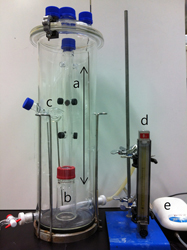
We report herein the crystal growth of ZnO nanoparticles by the foam fractionation method. In this study, the vertical column height of the foam was fixed and the velocity of the sparging air was varied, and the effect of foam flow rate on the synthesis of ZnO was investigated. The obtained ZnO consisted of aggregated platelets and had differing ultraviolet absorbances. The as-synthesized ZnO was hydrophobic because of the interaction between the anionic head groups of sodium dodecyl sulfate (SDS) and the ZnO under the precipitation conditions. The long chain of the SDS molecule was the cause of hydrophobicity. The contact angle of water was in the range of 95–105° for the obtained ZnO/SDS surface. The photocatalytic degradation efficiency of the as-synthesized (ZnO/SDS) and the calcined ZnO was investigated for methylene blue, and the calcined ZnO retained its activity even after three recycles.