Published online by Cambridge University Press: 01 July 2011
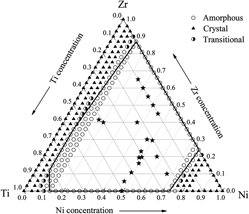
An atomistic scheme is developed based on constructed n-body potential to investigate the glass-forming composition region and atomic configurations in Ni–Zr–Ti system. The glass-forming ranges derived from the n-body potentials through molecular dynamics simulations for the binary Ni–Zr, Ni–Ti, Zr–Ti, and ternary Ni–Zr–Ti systems turns out to be very compatible with theoretical studies and experimental observations. Moreover, the coordination numbers (CNs), microchemical inhomogeneity parameter, and Honeycutt and Anderson pair analysis are also computed to exam the local atomic configurations during crystal-to-amorphous phase transition. It is found that average total CNs of amorphous phases are significantly larger compared with those in solid solution counterparts, owing to the increased fractions of CNs from 13 to 16. A tendency in forming the chemical short-range orders also exists in binary and ternary metallic glasses in the Ni–Zr–Ti system and icosahedra-related atomic configurations play important role in forming those orders.