Published online by Cambridge University Press: 14 July 2014
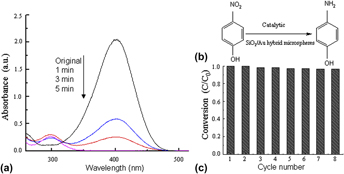
Au nanoparticles (Au NPs) have attracted much interest owing to their unique optical properties. In this paper, a facile process has been successfully developed to synthesize the SiO2/Au hybrid microspheres with a diameter of 200 nm via the galvanic replacement of SiO2/Ag hybrid microspheres and chlorauric acid (HAuCl4) solution. The as-prepared products were investigated by x-ray diffraction (XRD), field-emission scanning electron microscopy (FESEM, JEOL-6700F), and transmission electron microscopy (TEM, JEOL 3010), respectively. As expected, the as-prepared SiO2/Au hybrid microspheres show strong chemical stability and superior catalytic reduction of 4-nitrophenol (4-NP) to 4-aminophenol (4-AP). The SiO2/Au hybrid microspheres would be found widely used in wastewater treatment, catalytic reaction, bacteriostatic and bactericidal applications.