Published online by Cambridge University Press: 19 March 2019
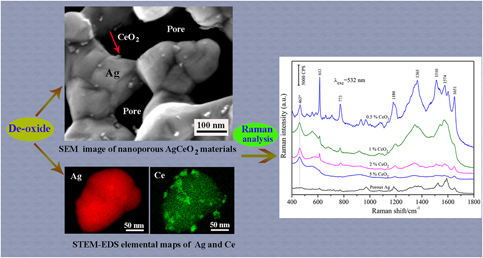
Noble metals combined with some oxides have synergetic contributions to surface-enhanced Raman scattering (SERS). In this work, a new method of de-oxide was proposed to prepare nanoporous metal based composites. Nanoporous Ag decorated with CeO2 nanoparticles was successfully prepared by decomposing Ag/CeO2/ZnO precursors in a 10 wt% NaOH aqueous solution. During the process of de-oxide, ZnO in the precursors could be removed completely and the nanoporous Ag/CeO2 nanocomposites with rough ligament surfaces were formed. The results indicated that the contents of CeO2 had significant influences on the microstructure and SERS performance of the prepared Ag/CeO2 materials. Using R6G and L-phenylalanine as probe molecules, the nanoporous Ag/CeO2(0.5%) substrates demonstrated a high enhancement factor of 1.2 × 108. The improved SERS performances were mainly attributed to the strong coupling effects between Ag ligament and CeO2 nanoparticle. This work would like to be interesting for the design of nanoporous composites for the application in the fields of SERS technology.