Published online by Cambridge University Press: 20 September 2019
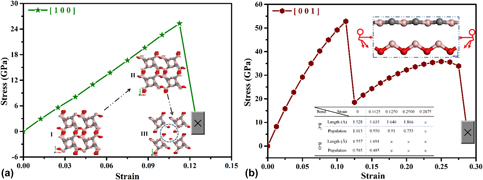
A novel tetragonal B2CO structure (tP16-B2CO), formed by strong covalent sp2–sp3 B–C and B–O bonds, was predicted with aid of an unbiased structure searching method. With the energy lower than those of previously proposed candidates, except oI16-B2CO, tP16-B2CO was identified as the thermodynamic metastable phase for B2CO compound. The elastic matrix and phonon dispersion spectra declare that tP16-B2CO is mechanically and dynamically stable. The electronic band structure calculation at ambient pressure and a series of high pressure has manifested the indirect semiconducting and band gap increases first and then decreases with pressure increases. The calculation of mechanical properties such as hardness and stress–strain relations of tP16 structure revealed its common hard nature with high hardness of 23.19 GPa and anisotropy with the max stress along [001] is far higher than that along [100].