Published online by Cambridge University Press: 20 September 2012
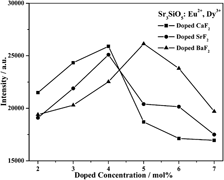
Yellow-emitting long afterglow phosphors Sr3−xSiO5, xMF2: Eu2+, Dy3+ (0 ≤ x ≤ 0.15, M: Ba, Sr, Ca) have been prepared by high-temperature solid-state reaction method followed with rapid cooling process. Photoluminescence measurement reveals that the main emission of the phosphors locates at 575 nm, corresponding to the 4f65d1–4f7 transition of Eu2+. The introduction of alkaline earth metal fluoride effectively enhances the luminescence intensity and prolongs the afterglow time. Especially, the afterglow of the Sr2.95SiO5, 0.05BaF2: Eu2+, Dy3+ phosphor can last for 12 h. Thermal luminescence measurement shows that the trap density of Sr3SiO5: Eu2+, Dy3+ phosphor can be adjusted by adding different alkaline earth metal fluorides, which offers a feasible way to improve the afterglow properties of silicate phosphors.