Published online by Cambridge University Press: 09 December 2015
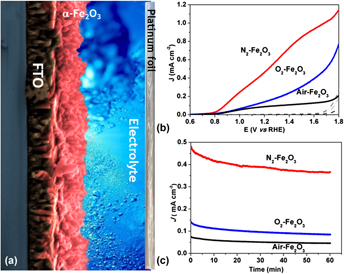
This work describes the effects of different atmospheres used during the thermal treatment of hematite films synthesized on transparent conductive substrates of fluorine-doped tin oxide by a newly reported wet chemical route assisted by microwave. The as-synthesized films were subjected to additional thermal treatment at 750 °C for 30 min in different gas flux (air, O2, and N2) to obtain a desirable phase and surface activation. A series of techniques were used to elucidate effects of each atmosphere used during the thermal treatment. The morphology of the films, as analyzed by top-view and cross-sectional scanning electron microscopy images, showed no significant changes and was composed of rods homogeneously distributed over the substrate, which covered the immersed area with a thickness between 98 and 100 nm. The photoelectrochemical response of the N2-hematite films was found to be 80 and 50% more efficient at 1.23 VRHE (reversible hydrogen electrode) than those of films produced in air and an O2 atmosphere. The photocurrent enhancement achieved by treatment in an oxygen-deficient atmosphere was attributed to the improvement of hematite catalytic activity, which produced a hematite–electrolyte interface favorable for water oxidation. Since an increase in the donor density by one order of magnitude was found for the N2-hematite films, a reduction of charge transfer resistance was expected in these films. However, the Nyquist plot analysis showed that the O2-hematite film had a lower charge transfer resistance. As a result, it is impossible to relate the photocurrent enhancement observed in N2-hematite film to electronic changes or vacancy formation, as previously reported in the literature. Indeed, by performing photoelectrochemical measurements in the presence of hole scavengers, it became clear that the major improvement caused by the oxygen-deficient atmosphere was in the catalytic activity efficiency of the hematite films for water oxidation. It was found that the oxygen-deficient atmosphere could improve the overall photoelectrochemical performance of the hematite by acting as a hole scavengers. This finding contrasts with a previous report, in which the use of an oxygen-deficient atmosphere during the phase transformation from akaganeite to hematite was found to enhance the photocurrent density by inducing an increased donor density caused by the formation of vacancies [Y. Ling et al., Angew. Chem., Int. Ed.51, 4074 (2012)].