Published online by Cambridge University Press: 14 June 2019
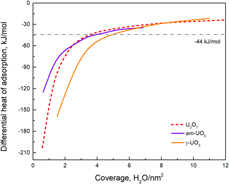
Enthalpies of water adsorption on amorphous and crystalline oxides and peroxides of uranium are reported. Despite substantial structural and computational research on reactions between actinides and water, understanding their surface interactions from the energetic perspective remains incomplete. Direct calorimetric measurements of hydration energetics of nano-sized, bulk-sized UO2, U3O8, anhydrous γ-UO3, amorphous UO3, and U2O7 were carried out, and their integral adsorption enthalpies were determined to be −67.0, −70.2, −73.0, −84.1, −61.6, and −83.6 kJ/mol water, with corresponding water coverages of 4.6, 4.5, 4.1, 5.2, 4.4, and 4.1 H2O per nm2, respectively. These energetic constraints are important for understanding the interfacial phenomena between water and U-containing phases. Additionally, this set of data also helps predict the absorption and desorption behavior of water from nuclear waste forms or used nuclear fuels under repository conditions. There are also underlying relations for water coverage among different U compounds. These experimentally determined data can be used as benchmark values for future computational investigations.
This author was an editor of this journal during the review and decision stage. For the JMR policy on review and publication of manuscripts authored by editors, please refer to http://www.mrs.org/editor-manuscripts/.